Nitroimidazole azole derivative, preparation method and application thereof
A compound and amino acid technology, applied in chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as unsafety, increased degree of degradation, and unknown toxicity of degraded impurities, and achieve good solubility, extended validity period, and superior stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
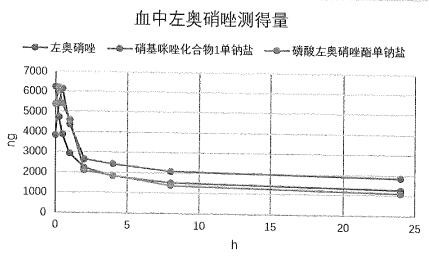
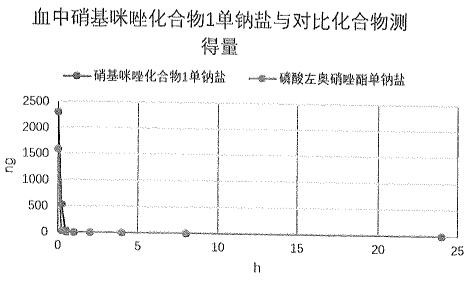
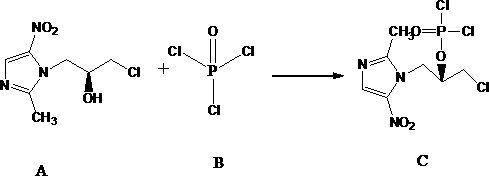
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of levonidazole phosphate
[0049] Weigh 10 g of left-ornidazole and place it in a dry 250 mL three-necked flask. Under nitrogen protection, add 100 mL of dichloromethane, cool down to 0-5°C, slowly add 6.56 g of phosphorus oxychloride, and finish adding in about 1 min. In-process HPLC was used to determine whether the reaction was complete.
[0050] Chromatographic conditions:
[0051] Mobile phase A: Potassium dihydrogen phosphate 6.8g / L, sodium heptanesulfonate 0.3g / L, adjust the pH value to 6.5 with triethylamine.
[0052] Mobile Phase B: Methanol
[0053] Detection wavelength: 321nm; Flow rate: l.0ml / min; Column temperature: 25°C; Sample concentration: 2mg / ml
[0054] Dilution medium: 0.1mol / L potassium dihydrogen phosphate
[0055] time (min) Mobile phase A (%) Mobile phase B (%) 0 85 15 5 85 15 10 75 25 20 75 25 25 55 45 45 55 45 50 85 15 60 85 15
[0056]Add the ...
Embodiment 2
[0057] Embodiment 2: the preparation of nitroimidazole compound 1
[0058] Take 10.0 g of levonidazole phosphate and place it in a dry 250 mL three-necked flask, add 100 ml of acetonitrile and stir evenly, add 10.5 g of 1-methylimidazole, 14.1 g of 2,2'-dithiodipyridine, triphenyl Base phosphorus 19.3g, react at room temperature for 1 hour, slowly add triethylamine phosphate 3.0g, during the reaction process, HPLC judges whether the reaction is complete.
[0059] Chromatographic conditions:
[0060] Mobile phase A: Potassium dihydrogen phosphate 6.8g / L, sodium heptanesulfonate 0.3g / L, adjust the pH value to 6.5 with triethylamine.
[0061] Mobile Phase B: Methanol
[0062] Detection wavelength: 321nm; Flow rate: l.0ml / min; Column temperature: 25°C; Sample concentration: 2mg / ml
[0063] Dilution medium: 0.1mol / L potassium dihydrogen phosphate
[0064] time (min) Mobile phase A (%) Mobile phase B (%) 0 85 15 5 85 15 10 75 25 20 75 25 ...
Embodiment 3
[0068] Embodiment 3: the preparation of nitroimidazole compound 1
[0069] Take 10.0g of levonidazole phosphate and 1.0g of triethylamine in a dry 100mL three-necked flask, add 50ml of dichloromethane and stir evenly, add N,N-carbonyldiimidazole at room temperature and stir for 2 hours, slowly add Triethylamine phosphate 3.0g, manganese chloride 2.4g and magnesium sulfate 3.0g, in the reaction process, HPLC judges whether the reaction is complete.
[0070] Chromatographic conditions:
[0071] Mobile phase A: Potassium dihydrogen phosphate 6.8g / L, sodium heptanesulfonate 0.3g / L, adjust the pH value to 6.5 with triethylamine.
[0072] Mobile Phase B: Methanol
[0073] Detection wavelength: 321nm; Flow rate: l.0ml / min; Column temperature: 25°C; Sample concentration: 2mg / ml
[0074] Dilution medium: 0.1mol / L potassium dihydrogen phosphate
[0075] time (min) Mobile phase A (%) Mobile phase B (%) 0 85 15 5 85 15 10 75 25 20 75 25 25 55...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com