Virus delivery culture medium
A culture medium and virus technology, applied in the field of virus culture medium, can solve the problems of easy breeding of bacteria, short storage time of virus preservation reagents, virus destruction, etc., to reduce the risk of drug resistance of pathogenic bacteria, superior compatibility and stability, Beneficial effect on antigenic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] An embodiment of the present invention provides a virus delivery medium, the virus delivery medium contains components at the following concentrations:
[0056] Hank'S balance solution, amino acid 1.3g / L, glycerin 20% (v / v), ProClin preservative 0.11% (v / v), bovine serum albumin 5g / L, phenol red 0.1g / L.
[0057] In the Hank'S balance solution, the following concentration components are included:
[0058] 4-Hydroxyethylpiperazineethanesulfonic acid 5g / L, NaCl: 8.22g / L, KCl: 2g / L, glucose 1g / L, disodium hydrogen phosphate 0.05g / L, CaCl 2 : 0.06g / L, MgCl 2 : 0.05g / L.
[0059] Contain the components of following concentration in the described ProClin antiseptic:
[0060] ProClin300 with a volume fraction of 0.1%, and ProClin500 with a volume fraction of 0.01%.
[0061] In addition, an embodiment of the present invention also provides a method for preparing the above-mentioned virus delivery medium, including the following steps:
[0062] Take solid state 4-hydroxyethyl...
Embodiment 2
[0064] In the embodiment of the present invention, the antigen detection performance verification of the virus delivery medium in Example 1 is carried out, specifically,
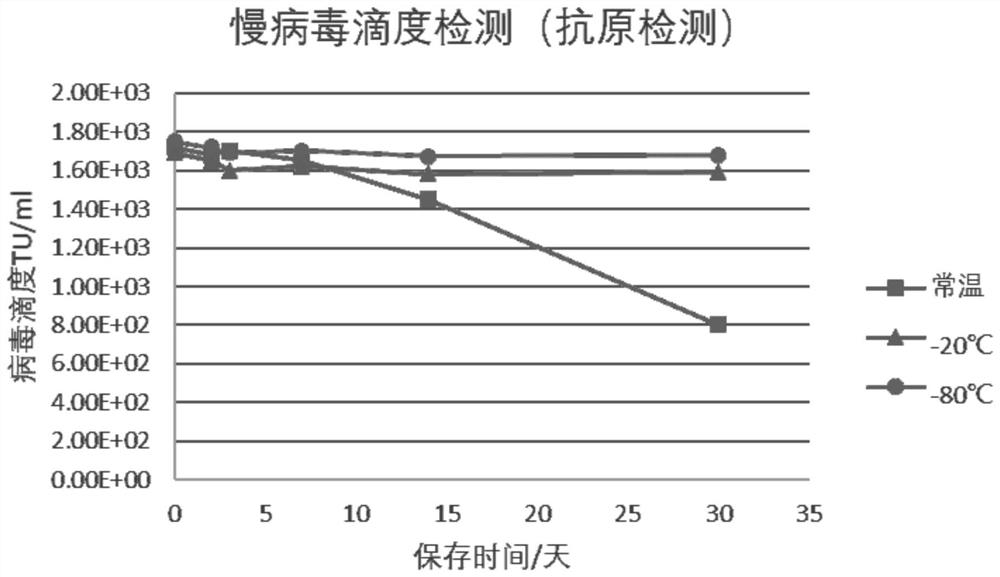
[0065] Take 9 parts of the virus delivery medium of the concentration components in Example 1, add the same concentration of lentivirus to each copy of the virus delivery medium, divide it into three groups of A, B, and C, and each group has 3 copies, and each copy of the virus delivery medium can be For testing more than 6 times. Groups A, B, and C were stored under different temperature conditions for virus delivery media. Group A was stored at room temperature, group B was stored at -20°C, and group C was stored at -80°C. Lentivirus detection was performed on day 0, day 2, day 3, day 7, day 14, and day 30, respectively. The lentivirus titer ELISA detection kit was used for detection, the OD value was measured by a microplate reader, the average value was taken, and the lentivirus titer was calculated usi...
Embodiment 3
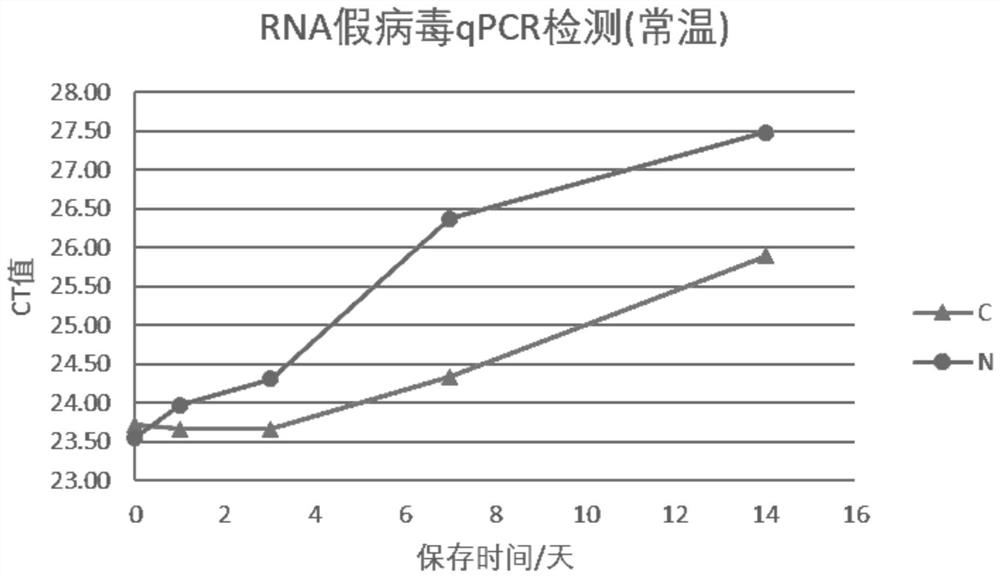
[0072] This embodiment verifies the nucleic acid detection performance of the virus transport medium of Example 1. Specifically, take 10 parts of the virus transport medium (C) of Example 1 of the present invention, add RNA pseudovirus, so that the concentration of each sample is 2000copies / mL. The culture medium samples added with RNA pseudoviruses were placed at room temperature for storage, and nucleic acid extraction and qPCR detection were performed on the 0th, 1st, 3rd, 7th, and 14th days of storage respectively.
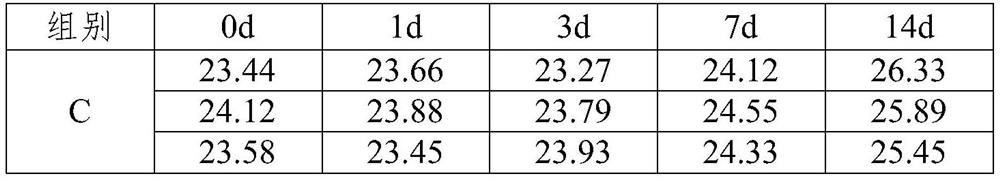
[0073] The test results are shown in Table 2:
[0074] Table 2 Example 1 The qPCR detection results of the virus delivery medium at different times
[0075]
[0076] Analysis of the experimental results in Table 2 shows that the RNA pseudoviruses preserved in the virus delivery medium of Example 1 of the present invention were stored at room temperature for 3 days, and the CT value had no significant change, and were stored at room temperature for one wee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com