Lentivirus adherent cell culture process
A technology of adherent cells and culture process, applied in the direction of virus, animal cells, culture process, etc., can solve the problems of increasing the risk of cell contamination, difficulty in quality inspection of transfection reagents, large batch differences, etc., to reduce cell contamination risk, shorten the time to pass, and simplify the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A lentivirus adherent cell culture process, which comprises the following steps:
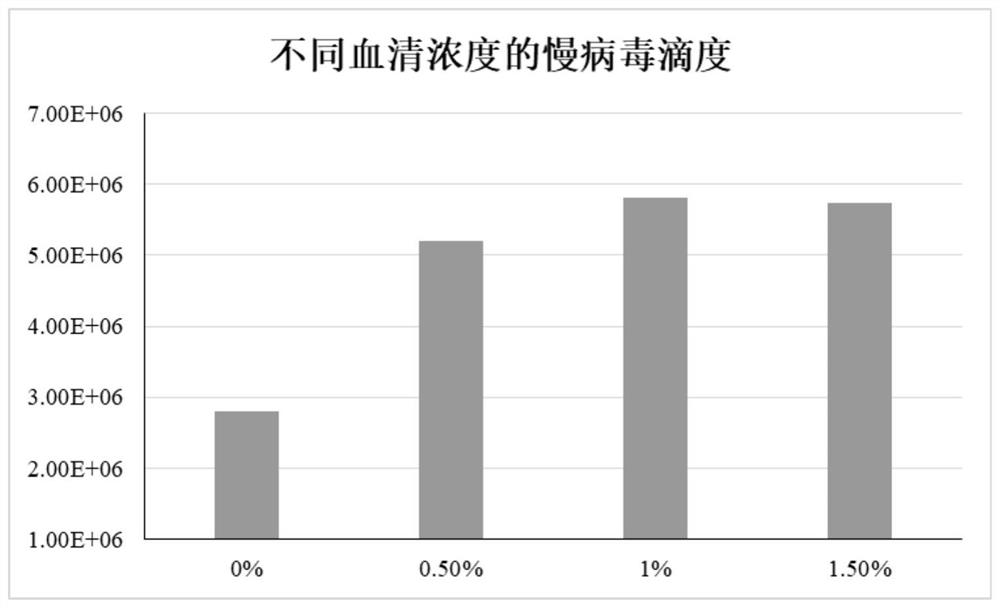
[0023] S1 HEK293T cells were adaptively cultured in a culture device equipped with VP-SFM medium and 0.5% fetal bovine serum.
[0024] S2 Step S1 Acclimate well-cultured HEK293T cells to establish a cell bank.
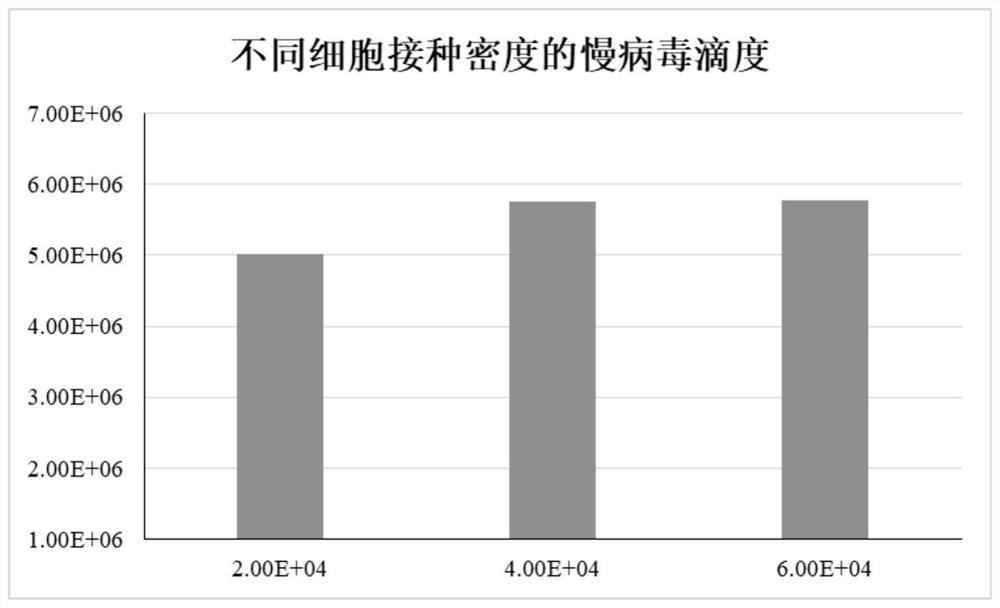
[0025] In S3, HEK293T cells in the logarithmic growth phase were inoculated into the cell factory, and the cell inoculation density was 2×10 4 / cm 2 , the cell factory is Nunc's CF-2 cell factory.
[0026] S4 In the four-plasmid system, HEK293T cells were transfected, PEIpro method was used to transfect HEK293T cells, and PEIpro: DNA was transfected at a ratio of 1:1.
[0027] After 50 hours of S5 transfection, the cell culture supernatant was harvested, and the cell viability was above 85%, and the titer of the transfection supernatant was 5×10 6 Lentiviral cells above TU / mL.
Embodiment 2
[0029] A lentivirus adherent cell culture process, which comprises the following steps:
[0030] S1 HEK293T cells were adaptively cultured in a culture device containing VP-SFM medium and 1% fetal bovine serum.
[0031] S2 Step S1 Acclimate well-cultured HEK293T cells to establish a cell bank.
[0032] In S3, HEK293T cells in the logarithmic growth phase were inoculated into the cell factory, and the cell inoculation density was 3×10 4 / cm 2 , the cell factory is Nunc's CF-2 cell factory.
[0033] S4 In the four-plasmid system, HEK293T cells were transfected, PEIpro method was used to transfect HEK293T cells, and PEIpro: DNA was transfected at a ratio of 1:1.
[0034] After 100 hours of S5 transfection, the cell culture supernatant was harvested, and the cell viability was over 90%, and the titer of the transfection supernatant was 5×10 6 Lentiviral cells above TU / mL.
Embodiment 3
[0036] A lentivirus adherent cell culture process, which comprises the following steps:
[0037] S1 HEK293T cells were adaptively cultured in a culture device containing VP-SFM medium and 1.5% fetal bovine serum.
[0038] S2 Step S1 Acclimate well-cultured HEK293T cells to establish a cell bank.
[0039] In S3, HEK293T cells in the logarithmic growth phase were inoculated into the cell factory, and the cell inoculation density was 4×10 4 / cm 2 , the cell factory is Nunc's CF-2 cell factory.
[0040] S4 In the four-plasmid system, HEK293T cells were transfected, PEIpro method was used to transfect HEK293T cells, and PEIpro: DNA was transfected at a ratio of 1:1.
[0041] After 100 hours of S5 transfection, the cell culture supernatant was harvested, and the cell viability was over 90%, and the titer of the transfection supernatant was 5×10 6 Lentiviral cells above TU / mL.
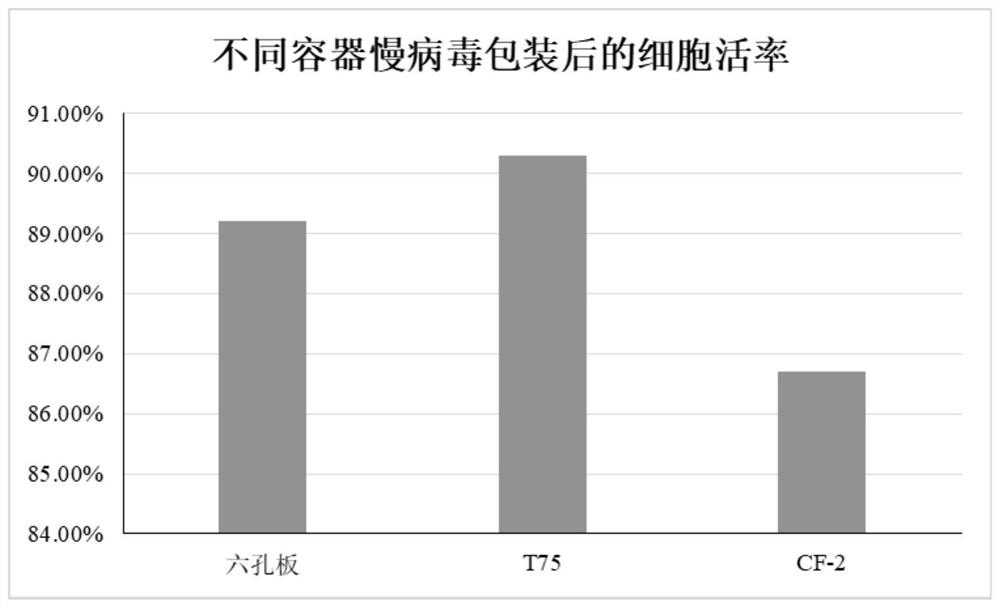
[0042] In the above schemes of Examples 1-3, in addition to using the CF-2 cell factory, six-well pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com