Catalytic ozonation catalyst as well as preparation method and application thereof
A technology of ozone catalytic oxidation and catalyst, which is applied in the field of water treatment, can solve the problems of poor stability, low activity and short life, and achieve the effects of stable performance, increased catalytic activity and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In this embodiment, a catalyst of the present invention is prepared, and its catalytic activity is tested.
[0056] The specific preparation method is:
[0057] γ-Al 2 o 3 The powder is placed in a vacuum oven for 3 hours in a vacuum;
[0058] With 0.741g 50% (mass fraction) manganese nitrate solution, 0.435g Co(NO 3 ) 2 ·6H 2 O, 0.303g Ce(NO 3 ) 2 ·6H 2 O, 0.365g Cu(NO 3 ) 2 ·3H 2 O, 0.505g Ca(NO 3 ) 2 4H 2 O was dissolved in 30ml of deionized water, stirred at room temperature for 2h;
[0059] 6g of processed γ-Al 2 o 3 Add the carrier to the metal salt solution and stir for 4h;
[0060] The impregnation solution and ammonia water (concentration 10%) were added dropwise to the same beaker, and the precipitation solution was continuously stirred during this process, and the dropping speed of the two solutions was controlled so that the pH value of the precipitation solution was controlled at 10. Continue to stir for 3h after the dropwise addition is c...
Embodiment 2
[0066] In this embodiment, a catalyst of the present invention is prepared, and its catalytic activity is tested.
[0067] The specific preparation method is:
[0068] γ-Al 2 o 3 The powder is placed in a vacuum oven for 3 hours in a vacuum;
[0069] 0.741g 50% manganese nitrate solution, 0.218g Co(NO 3 ) 2 ·6H 2 O, 0.151g Ce(NO 3 ) 2 ·6H 2 O, 0.2g Ni(CH 3 COO) 2 4H 2 O, 0.909g Fe(NO 3 ) 2 9H 2 O, 0.505g Ca(NO 3 ) 2 4H 2 O was dissolved in 30ml of deionized water, stirred at room temperature for 2h;
[0070] 6g of processed γ-Al 2 o 3 Add the carrier to the metal salt solution and stir for 4h;
[0071] The impregnation solution and ammonia water (concentration 10%) were added dropwise to the same beaker, and the precipitation solution was continuously stirred during this process, and the dropping speed of the two solutions was controlled so that the pH value of the precipitation solution was controlled at 10. Continue to stir for 3h after the dropwise addi...
Embodiment 3
[0077] In this embodiment, a catalyst of the present invention is prepared, and its catalytic activity is tested.
[0078] The specific preparation method is the same as in Example 1, the difference is only: adopt 0.741g 50% (mass fraction) manganese nitrate solution, 0.435g Co(NO 3 ) 2 ·6H 2 O, 0.303g Ce(NO 3 ) 2 ·6H 2 O, 0.365g Cu(NO 3 ) 2 ·3H 2 O, 0.253g Ca(NO 3 ) 2 4H 2 O.
[0079] Obtain catalyst Mn(3%)-Co(2%)-Ce(2%)-Cu(2%)-Ca(1%) / γ-Al 2 o 3 .
[0080] Catalyst activity test:
[0081] COD cr 100ml of industrial waste water of 295mg / L (pH value is 6.5), with ozone as oxidant continuous dosing, gas flow rate is 62ml / min, O 3 The gas phase concentration is 27mg / L, and the catalyst consumption is 5g.
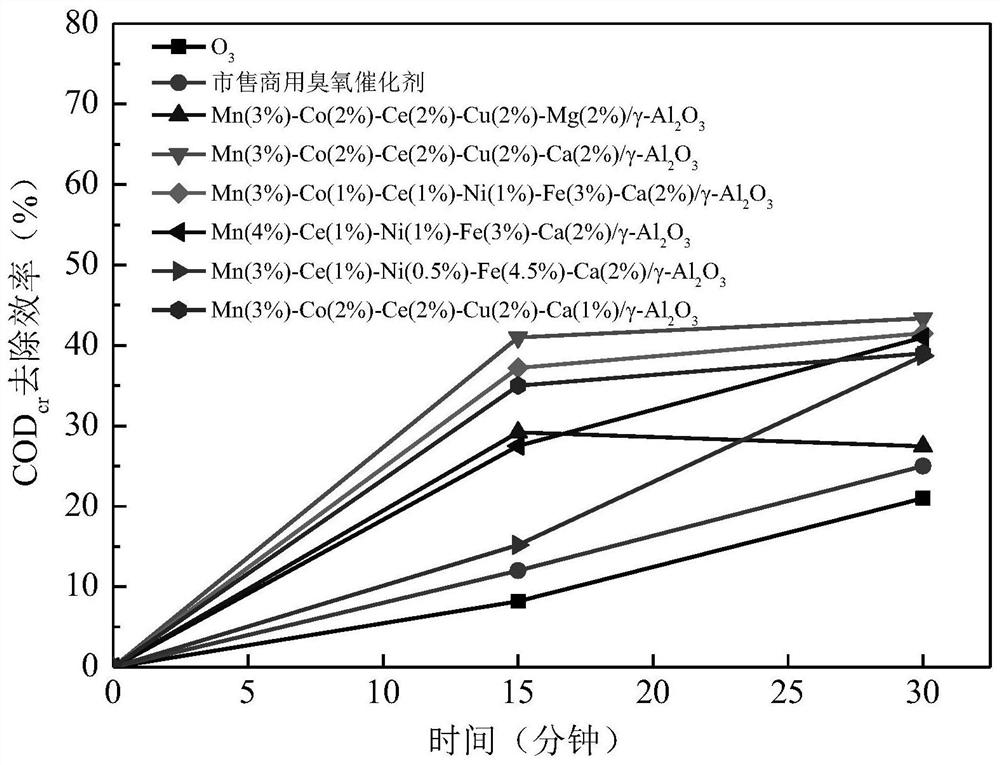
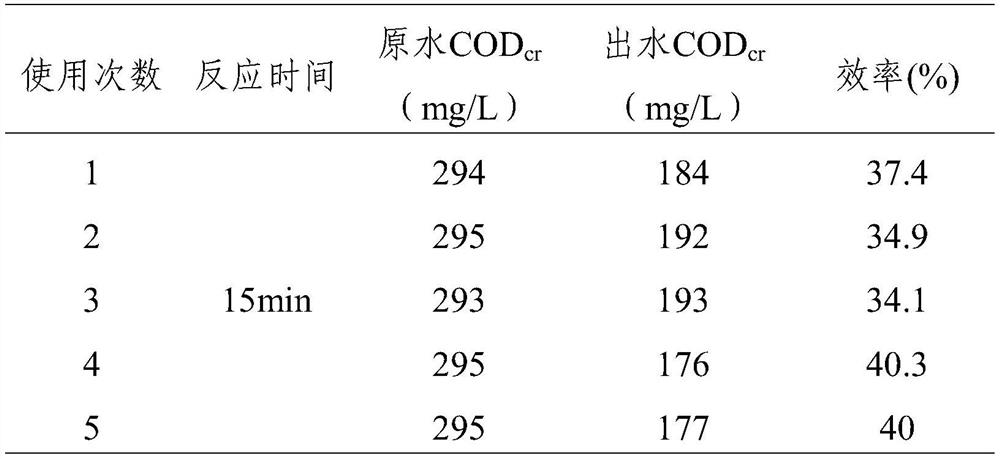
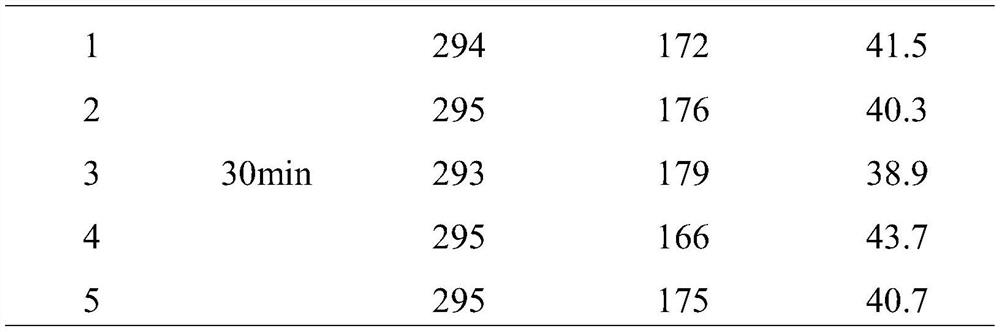
[0082] When reacting for 15 minutes, the COD of the wastewater by the catalyst cr The removal rate was 35%, and the removal rate was 39% at 30 minutes. The catalyst works quickly and the catalytic efficiency is stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com