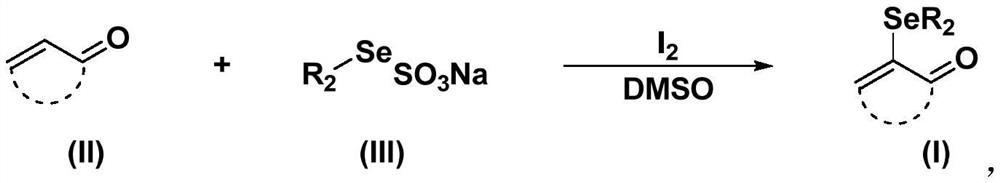Method for synthesizing alpha-seleno-alpha, beta-unsaturated carbonyl compound from organic sodium selenosulfate
A carbonyl compound and sodium sulfosulfate technology, applied in organic chemistry and other fields, can solve problems such as high environmental hazards, increased costs, and small substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 0.073g (0.5mmol) benzylidene acetone, 0.2055g (0.75mmol) sodium benzylselenosulfate, 0.0254g (0.1mmol) iodine and 2mL (25mmol) dimethylsulfoxide to a 35mL thick-walled pressure-resistant tube . The reaction was stirred at 70°C for 8h. After the reaction, the reaction solution was diluted with 20 mL of water, extracted three times with 15 mL of ethyl acetate, the organic phases were combined, washed with saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate, the volume ratio of the two was 10:1) to obtain 0.1153 g of α-benzylselenyl-benzylidene acetone, with a yield of 73% .
[0029] α-Benzylidene-benzylideneacetone 1 H NMR and 13 C NMR data are as follows.
[0030] 1 H NMR (500MHz, Chloroform-d) δ7.53(d, J=15.9Hz, 1H), 7.48–7.45(m, 2H), 7.34–7.28(m, 4H)...
Embodiment 2
[0033] Add 0.073g (0.5mmol) chromone, 0.195g (0.75mmol) sodium phenylselenosulfate, 0.0254g (0.1mmol) iodine and 2mL (25mmol) dimethylsulfoxide into a 35mL thick-walled pressure-resistant tube. The reaction was stirred at 70°C for 8h. After the reaction, the reaction solution was diluted with 20 mL of water, extracted three times with 15 mL of ethyl acetate, the organic phases were combined, washed with saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate, the volume ratio of which was 20:1) to obtain 0.1042 g of α-phenylselenyl-chromone with a yield of 69%.
[0034] α-Phenylselenyl-chromone 1 H NMR and 13 C NMR data are as follows.
[0035] 1 H NMR (500MHz, Chloroform-d) δ8.23 (d, J = 8.0Hz, 1H), 7.88 (d, J = 0.9Hz, 1H), 7.70-7.56 (m, 3H), 7.41 (dd, J = 9.7,8.2Hz...
Embodiment 3
[0038] This embodiment is basically the same as Embodiment 1, the only difference is that the reaction temperature is 60°C. The yield was 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com