Hydrogel of sulfydryl-modified high-molecular compound as well as preparation method and application of hydrogel
A technology of polymer compounds and hydrogels, which can be used in medical preparations, pharmaceutical formulations, drug delivery and other directions of non-active ingredients, and can solve the problems of slow speed, high price and affecting hydrogels.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
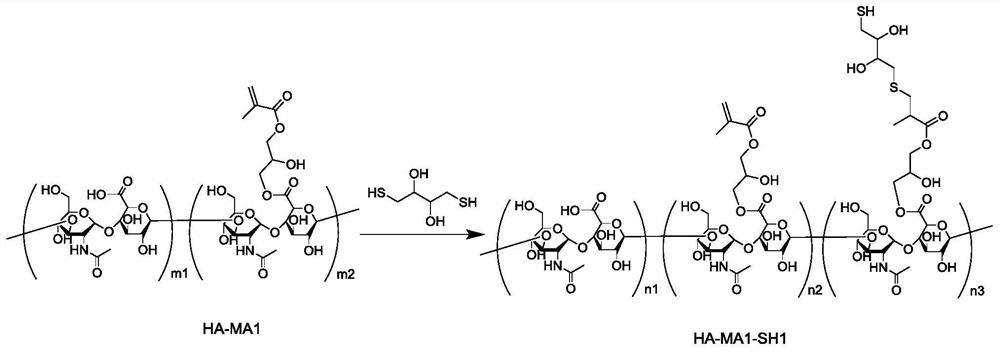
[0202] [Preparation method of mercapto-modified polymer compound]
[0203] As mentioned above, the present invention provides a method for preparing the above-mentioned mercapto-modified polymer compound, which comprises the following steps:
[0204] 1) The structure contains -COOH, -NH 2 The acrylation step of at least one polymer compound in -OH, that is, the -COOH, -NH contained in the structure of the polymer compound 2 , at least one of -OH is directly or indirectly linked to the following groups:
[0205]
[0206] R 1 , R 2 and R 3 The definition of is the same as before; * represents the connection point;
[0207] Alternatively, a polymer compound structurally containing at least one of the acrylate group shown in the above formula a, the acrylamide group shown in the above formula b, and the acryloyl group shown in the above formula c is directly used as the reaction raw material;
[0208] 2) At least one of the polymer compounds obtained in step 1) and the po...
preparation example 1
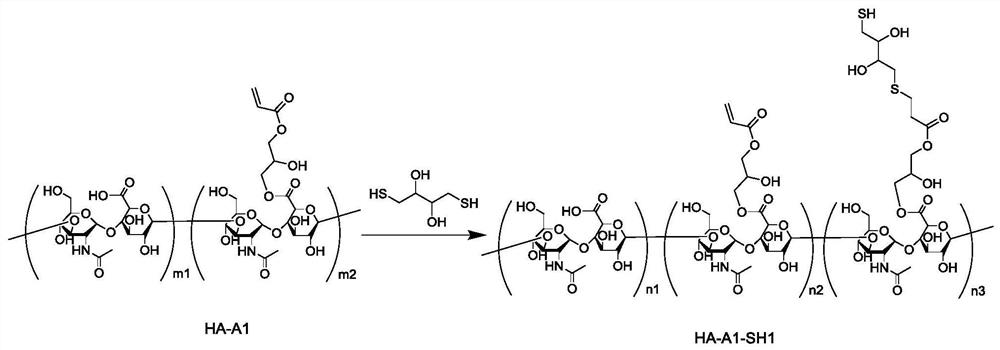
[0305] Preparation Example 1 Synthesis of acrylate-modified hyaluronic acid (HA-A1 for short)
[0306] Add 1 gram of hyaluronic acid (purchased from Huaxi Freda Company, its weight average molecular weight is about 300kDa), 50 milliliters of deionized water, 50 milliliters of dimethylformamide, 12 milliliters of triethylamine in a 200 milliliter beaker, 14 ml glycidyl acrylate. After stirring at room temperature until homogeneous and transparent, the stirring was continued for 48 hours. 300 ml of acetone was added, resulting in a large amount of white precipitate. The precipitate obtained by centrifugation was dissolved in 100 ml of deionized water to obtain a colorless transparent solution. The above solution was put into a dialysis bag (molecular weight cut-off 8kDa), dialyzed with 5 liters of deionized water for 5 days, and changed the water twice a day. Finally, the solution in the dialysis bag was collected and freeze-dried to obtain 921 mg of white flocculent solid, n...
preparation example 2
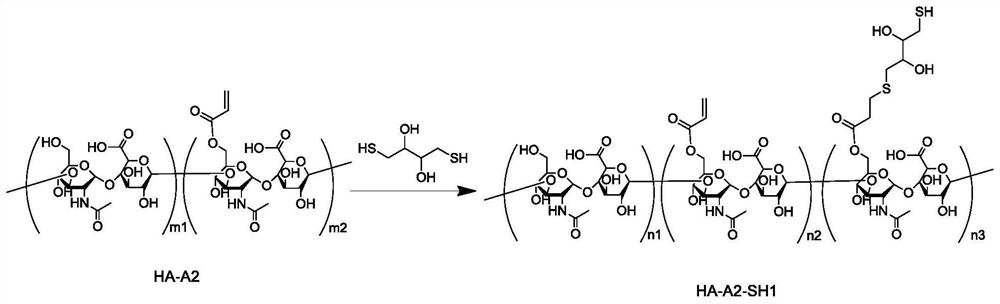
[0309] Preparation Example 2 Synthesis of acrylate-modified hyaluronic acid (HA-A2 for short)
[0310] Add 1 gram of hyaluronic acid (purchased from Huaxi Freda Company, its weight average molecular weight is about 400kDa), 50 milliliters of deionized water, 50 milliliters of dimethylformamide, 6.3 grams of acrylic anhydride in a 200 milliliter beaker, stir dissolve. The pH of the solution was maintained at 8±0.5 with 1 mole per liter of NaOH, and stirring was continued for 24 hours. 300 ml of acetone was added, resulting in a large amount of white precipitate. The precipitate obtained by centrifugation was dissolved in 100 ml of deionized water to obtain a colorless transparent solution. The above solution was packed into a dialysis bag (molecular weight cut-off 8kDa, Spectrum), dialyzed with 5 liters of deionized water for 5 days, and changed the water twice a day. Finally, the solution in the dialysis bag was collected and freeze-dried to obtain 789 mg of white flocculen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com