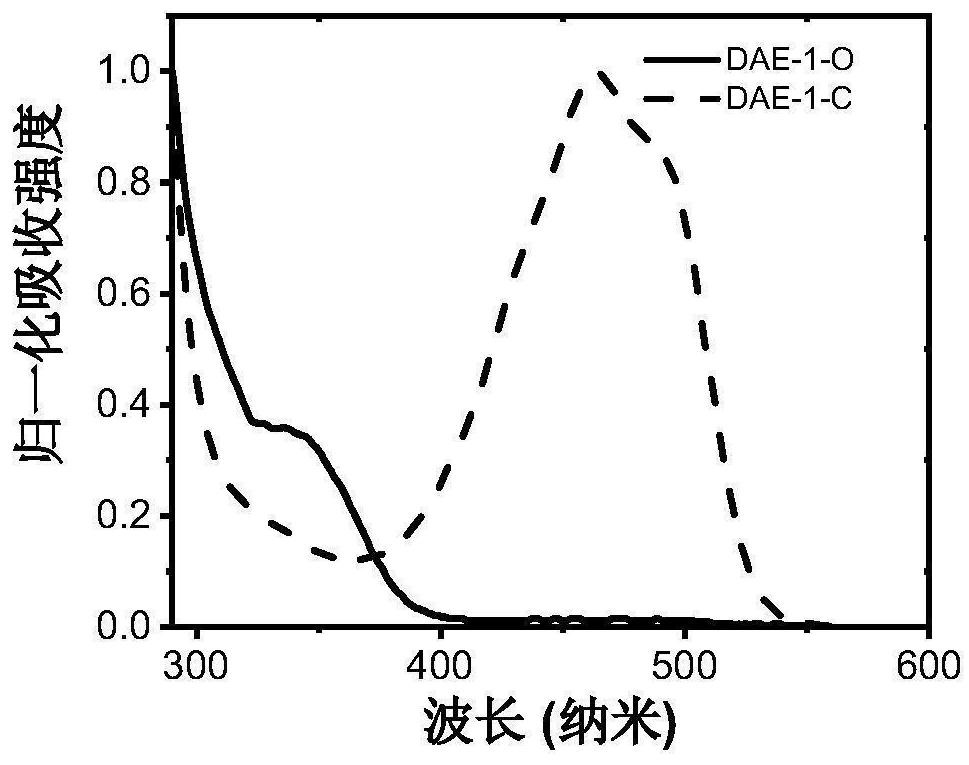
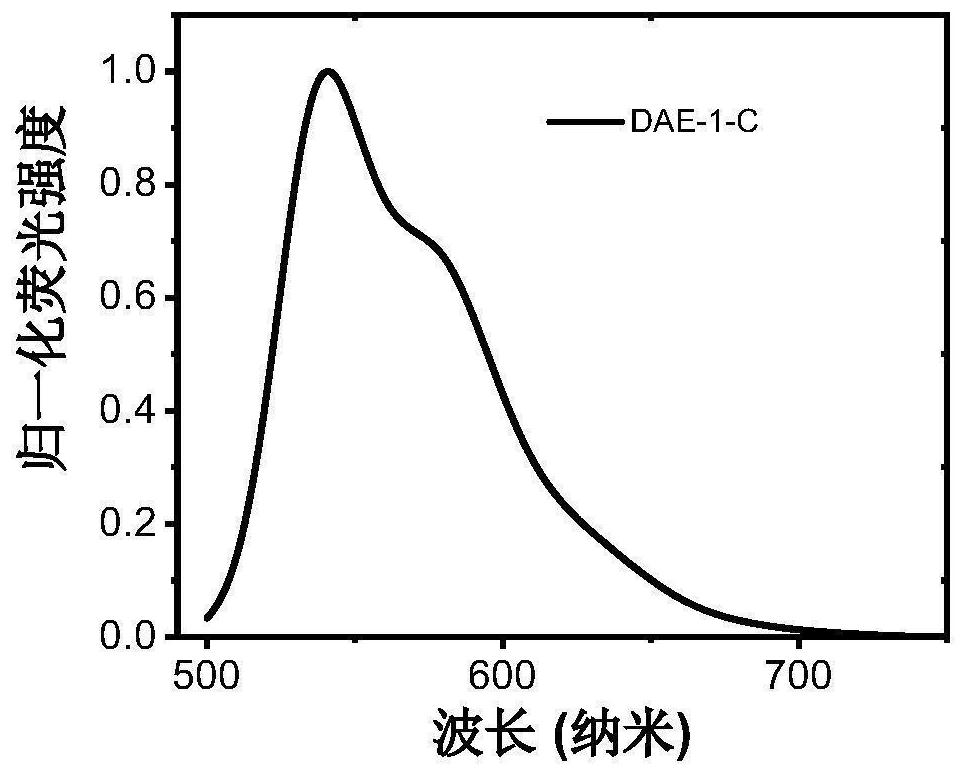
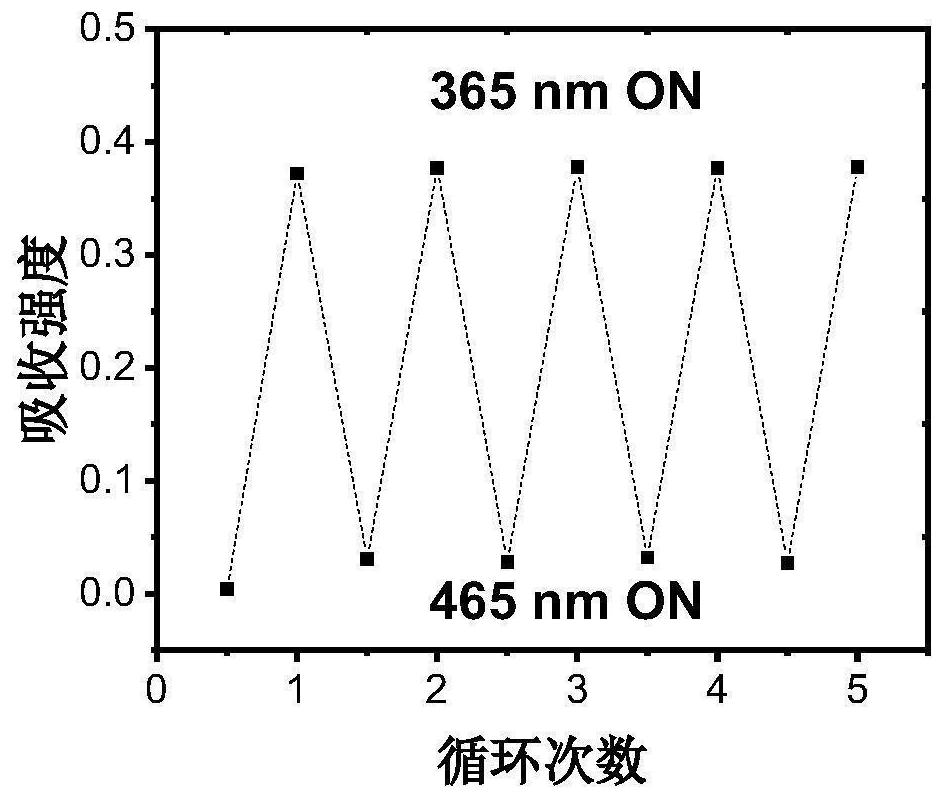
Photochromic diaryl ethylene compound with up-conversion luminescence property and application thereof
A diarylethene and photochromic technology, applied in organic chemistry, color-changing fluorescent materials, chemical instruments and methods, etc., to achieve the effect of eliminating background noise, strong tissue penetration ability, and good reversible optical switch performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Synthesis of Photochromic Diarylethene Compound DAE-1 with Upconversion Luminescent Properties
[0103] The synthetic route is:
[0104]
[0105] (1) Synthesis of Intermediate 1
[0106] Under an argon atmosphere, slowly add n-butyllithium hexane solution (53mL, 1.6M) dropwise into a tetrahydrofuran solution (100mL) containing benzothiophene (10g, 75mmol) at -78°C. The reaction was continued at °C for 1 h, then ethyl bromide (3.2 mL, 84 mmol) was slowly added dropwise at -78 °C. After dropping, slowly return to room temperature, and continue to react for 12h. After the reaction was complete, add saturated aqueous sodium chloride solution (50mL) to quench the reaction, remove the solvent under reduced pressure, then add saturated aqueous sodium chloride solution (100mL), extract with petroleum ether (100mL), use sodium sulfate to dry the organic phase, spin off Solvent, using petroleum ether for column chromatography, isolated intermediate 1 (11 g, yield 82%).
[...
Embodiment 2
[0121] Synthesis of Photochromic Diarylethene Compound DAE-2 with Upconversion Luminescent Properties
[0122] The synthetic route is:
[0123]
[0124] Steps (1), (2) and (3) of this embodiment are the same as in Embodiment 1.
[0125] Synthesis of Photochromic Diarylethene Compound DAE-2 with Upconversion Luminescent Properties
[0126] Weigh intermediate 4 (1.72g), 4-(2-bromoethoxy)phenylboronic acid (0.5g), potassium carbonate (0.6g), Pd 2 (dba) 3 (0.3g) was dissolved in dioxane (10mL), tricyclohexylphosphonium toluene solution (0.8mL, 18wt%) was added, refluxed under argon atmosphere for 12h, cooled to room temperature after the reaction was completed, the solvent was rotated, extracted with chloroform , dry the organic phase with sodium sulfate, spin off the solvent and perform column chromatography (developing solvent: petroleum ether / ethyl acetate volume ratio = 20:1) to obtain a light yellow solid (0.15g, yield 83%).
[0127] NMR and MS data of photochromic dia...
Embodiment 3
[0129] Synthesis of Photochromic Diarylethene Compound DAE-3 with Upconversion Luminescent Properties
[0130]
[0131] Steps (1), (2) and (3) of this embodiment are the same as in Embodiment 1.
[0132] Weigh intermediate 4 (0.86g), thienothiophene boronic acid (0.4g), potassium carbonate (0.4g), Pd 2 (dba) 3 (0.2g) was dissolved in dioxane (10mL), tricyclohexylphosphonium toluene solution (0.4mL, 18wt%) was added, refluxed for 4h under argon atmosphere, cooled to room temperature after the reaction was completed, the solvent was spun off, and extracted with chloroform , dry the organic phase with sodium sulfate, spin off the solvent and perform column chromatography (developing solvent: petroleum ether / ethyl acetate volume ratio = 20:1) to obtain an orange solid (0.3 g, yield 70%).
[0133] NMR and MS data of photochromic diarylethene compound DAE-3 with upconversion luminescence properties: 1 HNMR (400MHz, chloroform-d) δ7.85-7.92(m, 2H), 7.74-7.76(m, 1.3H), 7.52-7.58...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com