Modified lignin as well as preparation method and application thereof in rubber composite material
A lignin modification technology, applied in the field of rubber composite materials, can solve problems such as poor reinforcement effect, limited lignin application, and weak binding effect, so as to improve compatibility and affinity, inhibit self-aggregation tendency, The effect of reducing the polarity of molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
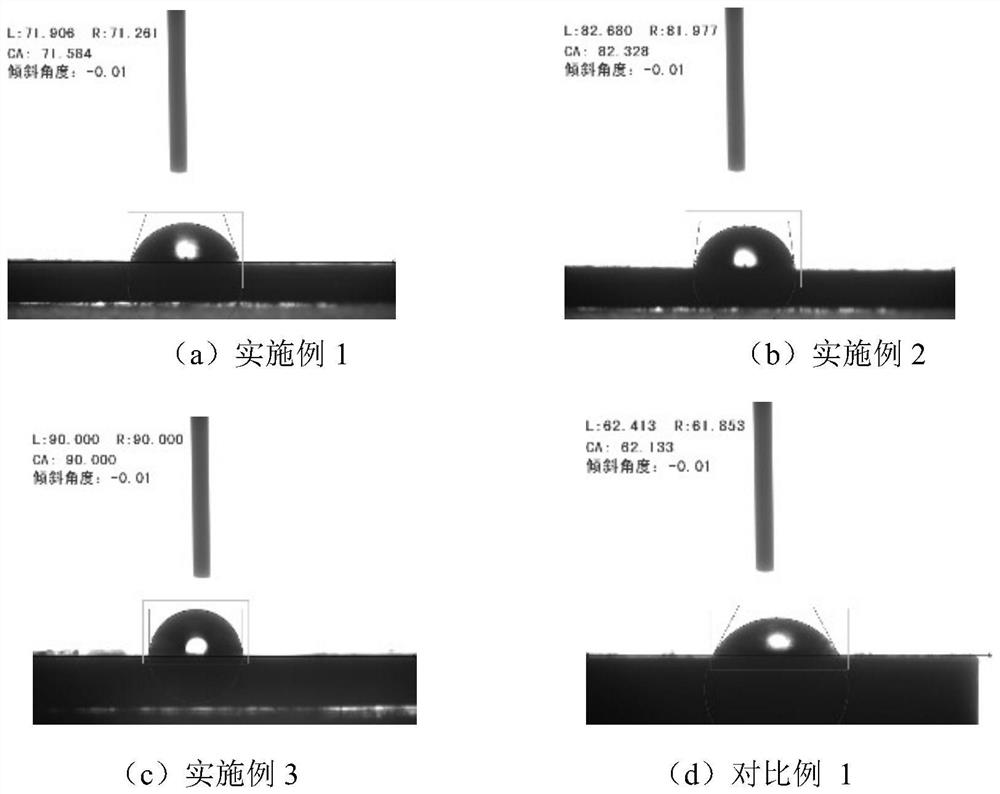
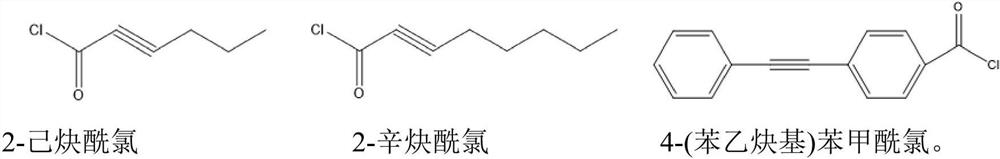
[0048] (1) Add alkali lignin and propioyl chloride into DMSO at a mass ratio of 100:18, the mass concentration of alkali lignin is 0.2g / mL, react at 60°C for 6 hours, cool to room temperature and add appropriate amount of deionized water to obtain Alkynylated lignin is precipitated, washed with deionized water until neutral, and vacuum-dried at 90°C for 24 hours to obtain alkynylated lignin;
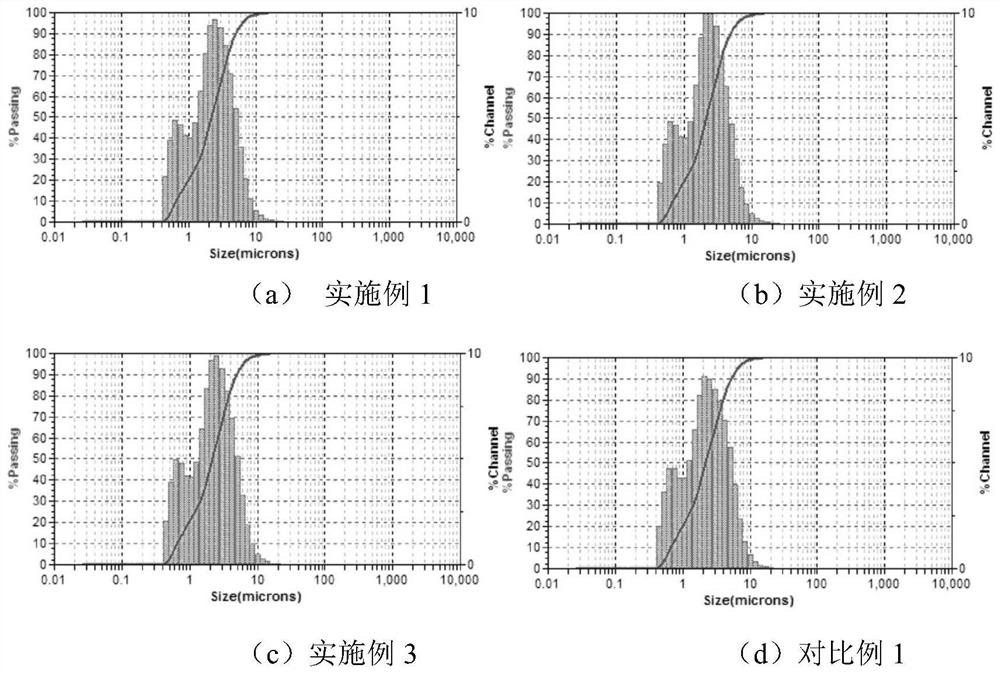
[0049] (2) the alkynylated lignin obtained in step (1) is ground to d by a jet mill 50 About 2.2μm;
[0050] (3) Accurately weigh 6.0g of the alkynylated lignin obtained in step (2), add 40.0g of natural rubber, 14.0g of carbon black N330, 2.0g of zinc oxide, and 1.2g of stearic acid into the internal mixer successively, and heat at 100°C 15 minutes of banburying under the same conditions;
[0051] (4) Put the mixed rubber obtained in the step (3) into the rolling mill, add 1.0 g of sulfur and 0.24 g of benzothiazole disulfide in turn, mix for 10 minutes at 20 ° C, and pass through 5 t...
Embodiment 2
[0053] (1) Add alkali lignin and propioyl chloride into DMSO at a mass ratio of 100:36, the mass concentration of alkali lignin is 0.2g / mL, react at 60°C for 6 hours, cool to room temperature and add appropriate amount of deionized water to obtain Alkynylated lignin is completely precipitated, washed with deionized water until neutral, and vacuum-dried at 90°C for 24 hours to obtain alkynylated lignin;
[0054] (2) the alkynylated lignin obtained in step (1) is ground to d by a jet mill 50 About 2.2μm;
[0055] (3) Accurately weigh 6.0g of the alkynylated lignin obtained in step (2), add 40.0g of natural rubber, 14.0g of carbon black N330, 2.0g of zinc oxide, and 1.2g of stearic acid into the internal mixer successively, and heat at 100°C 15 minutes of banburying under the same conditions;
[0056] (4) Put the mixed rubber obtained in the step (3) into the rolling mill, add 1.0 g of sulfur and 0.24 g of benzothiazole disulfide in turn, mix for 10 minutes at 20 ° C, and pass ...
Embodiment 3
[0058] (1) Add alkali lignin and propioyl chloride into DMSO at a mass ratio of 100:54, the mass concentration of alkali lignin is 0.2g / mL, react at 60°C for 6 hours, cool to room temperature and add appropriate amount of deionized water until Alkynylated lignin is precipitated, washed with deionized water until neutral, and vacuum-dried at 90°C for 24 hours to obtain alkynylated lignin;
[0059] (2) the alkynylated lignin obtained in step (1) is ground to d by jet milling 50 About 2.2μm;
[0060] (3) Accurately weigh 6.0g of the alkynylated lignin obtained in step (2), add 40.0g of natural rubber, 14.0g of carbon black N330, 2.0g of zinc oxide, and 1.2g of stearic acid into the internal mixer successively, and heat at 100°C 15 minutes of banburying under the same conditions;
[0061] (4) Put the mixed rubber obtained in the step (3) into the rolling mill, add 1.0 g of sulfur and 0.24 g of benzothiazole disulfide in turn, mix for 10 minutes at 20 ° C, and pass through 5 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com