Gene/drug composite lipid preparation and preparation method and application thereof
A technology for compounding lipids and preparations, which can be used in drug combinations, gene therapy, pharmaceutical formulations, etc., and can solve problems such as unsatisfactory results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
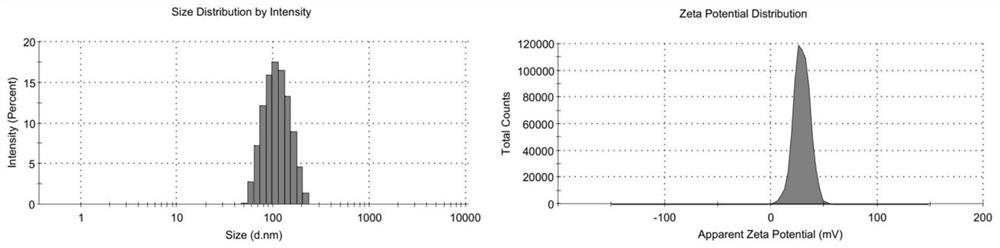
[0075] Preparation and Characterization of Example 1 Gene / Drug Composite Lipid Carrier
[0076] (1) Reagent and lipid stock solution: DOTAP / Chol / DPPC stock solution: 25mg / mL, weigh 100mg DOTAP / Chol / DPPC dissolved in 4mL chloroform; DMG-PEG2000 stock solution: 5mg / mL, weigh 20mgDMG - PEG2000 dissolved in 4mL chloroform; ammonium sulfate solution: 200mM, prepared by weighing 1.057g solid ammonium sulfate powder and dissolving it in 40mL ultrapure water;
[0077] (2) Pipette an appropriate amount of DOTAP / Chol / DPPC / DMG-PEG2000 stock solution into a round-bottomed flask with a micro-syringe needle, the molar ratio is 50:40:9:1, add an appropriate amount of chloroform, and place in a 50°C water bath Rotary evaporation to remove organic solvent;
[0078] (3) drying up the organic solvent remaining in the round-bottomed flask of step (2) with nitrogen to make lipid film;
[0079] (4) adding an appropriate amount of 200mM ammonium sulfate solution preheated to 50°C into the lipid fi...
Embodiment 2
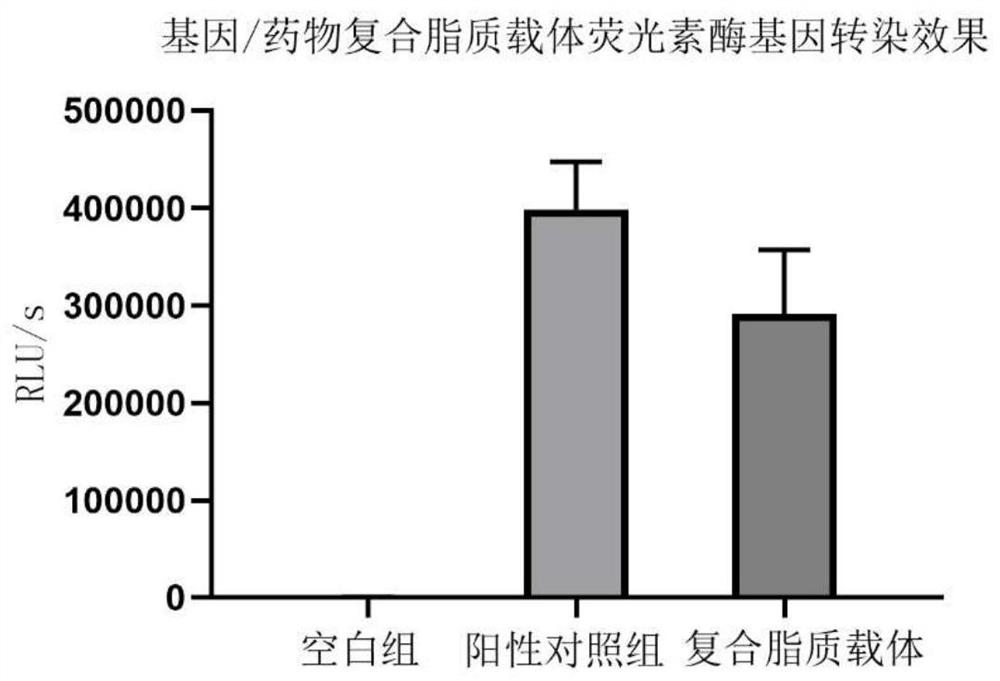
[0084] The luciferase gene transfection effect evaluation of embodiment 2 gene / drug composite lipid carrier
[0085] (1) 24 hours in advance, mouse hepatic stellate cells (JS1) were treated with 1*10 4 Seed into a 96-well plate at the density of each well, add 100uL DMEM complete medium containing 10% FBS to each well, and enter the next step after the cell confluency reaches 80%;
[0086] (2) Blank cationic liposomes were co-incubated with pGL3 plasmid at a nitrogen-to-phosphorus ratio of 4:1. Add about 250ng of plasmid to each well, add DMEM medium to a total volume of 100uL per well, and place in a 37°C incubator without serum transfer. Change the medium after 4-6 hours of staining, add complete medium and continue to culture for 24 hours;
[0087] (3) 24 hours after transfection, wash with pre-cooled PBS to remove residual medium, add 50uL cell lysate to each well, lyse at room temperature for 10 minutes, and centrifuge at 4,000rpm in an orifice centrifuge for 2 minutes; ...
Embodiment 3
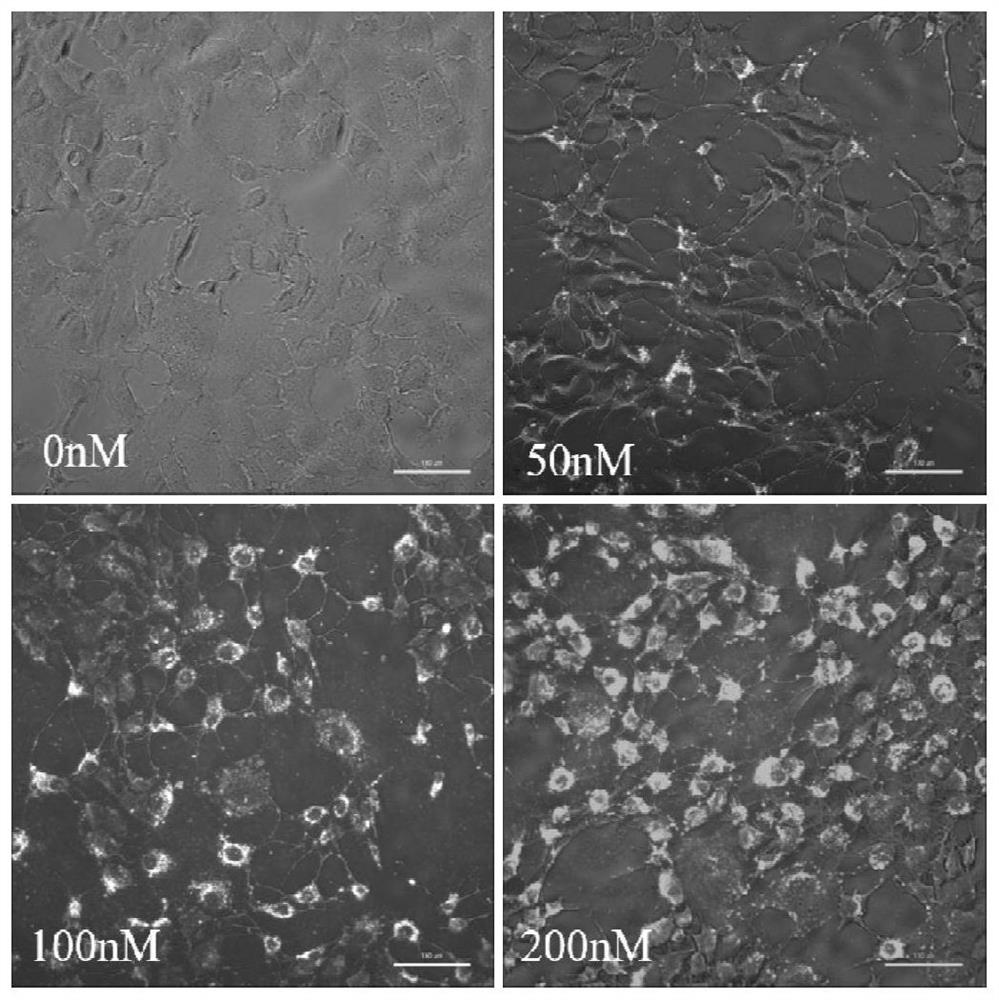
[0090] Example 3 Efficiency Evaluation of Gene / Drug Composite Lipid Carrier Importing Cy5-siRNA into Hepatic Stellate Cells
[0091] (1) 24 hours in advance of JS1 cells with 1*10 4 Seed into a 96-well plate at the density of each well, add 100uL DMEM complete medium containing 10% FBS to each well, and enter the next step after the cell confluency reaches 80%;
[0092] (2) Blank cationic liposomes were incubated with cy5-siRNA at a nitrogen-to-phosphorus ratio of 4:1 in the dark, and three concentrations of siRNA were set, namely 50nM, 100nM, and 200nM, and DMEM medium was added to make the total volume of each well 100uL. Serum-free transfection in a 37°C incubator;
[0093] (3) Change the medium after 4-6 hours of transfection, wash with pre-warmed PBS three times, and remove unbound free siRNA;
[0094] (4) The transfection efficiency was observed under a fluorescence microscope, and the images were converted into grayscale images using ImageJ software.
[0095] The res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com