PSMA inhibitor, compound as well as preparation method and application of compound
A compound and inhibitor technology, applied in the field of compounds and their preparation, PSMA inhibitors, can solve the problems of low sensitivity, slow clearance in vivo, affecting the diagnosis of liver metastases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the synthesis of I-112 (2-pyridine-cyclohexylmethylamine-DOTA-PSMA)
[0062] Take 100 mg of Fmoc-Glu-urea-Lys-resin (tert-butyl protected glutamic acid urea-lysine resin) (0.22 mmol / g) in a solid-phase synthesis tube. DCM washed (3×5min×2mL), DMF washed (3×5min×2mL). De-Fmoc using 20% piperidine in DMF (1 x 2 min x 2 mL, 2 x 10 min x 2 mL), followed by washing with DMF (6 x 1 min x 2 mL).
[0063] Take Fmoc-3-(2-pyridine)-D-alanine (3M, 0.06mmol, 26.2mg), HBTU (0.072mmol, 27mg), HOBt (0.072mmol, 10mg), DIPEA (0.15mmol, 25μL) in 3 mL DMF, room temperature for 15 minutes. The above activated 2-pyridine-alanine was added to the washed resin and reacted for 1 hour under nitrogen. Wash with DMF (6×1 min×2 mL). De-Fmoc using 20% piperidine in DMF (1 x 2 min x 2 mL, 2 x 10 min x 2 mL), followed by washing with DMF (6 x 1 min x 2 mL).
[0064] Take trans-4-(Fmoc-aminomethyl)cyclohexanecarboxylic acid (3M, 0.06mmol, 23mg), HBTU (0.072mmol, 27mg), HOBt (0.0...
Embodiment 2
[0067] Embodiment 2: the synthesis of I-113 (3 pyridine-cyclohexylamine-DOTA-PSMA)
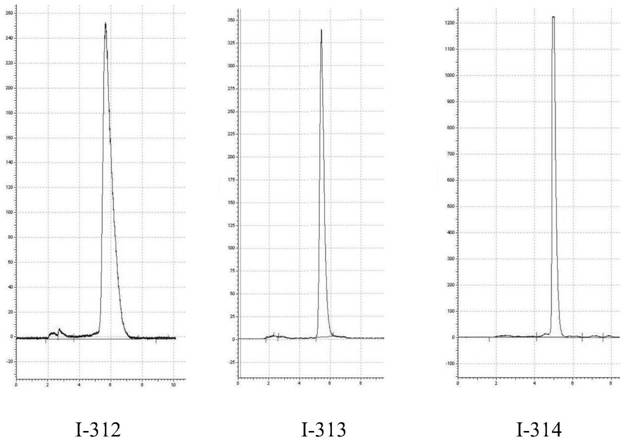
[0068] The Fmoc-3-(2-pyridine)-D-alanine in Example 1 is replaced by Fmoc-3-(3-pyridine)-D-alanine, and the rest remain unchanged, and the final synthesis product is passed through HPLC Purified to obtain a white final product: 3-pyridine-cyclohexylmethylamine-DOTA-PSMA, mass spectrogram see figure 2 . MS: [M+H] (m / z=993.6)
Embodiment 3
[0069] Embodiment 3: the synthesis of I-114 (4-pyridine-cyclohexylamine-DOTA-PSMA)
[0070] The Fmoc-3-(2-pyridine)-D-alanine in Example 1 is replaced by Fmoc-3-(4-pyridine)-D-alanine, and the rest remain unchanged, and the final synthesis product is passed through HPLC Purified to obtain a white final product: 4-pyridine-cyclohexylmethylamine-DOTA-PSMA, mass spectrogram see image 3 . MS: [M+H] (m / z=991.99)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com