Bispecific antibody capable of combining PD-L1 and TGF-beta of mouse as well as preparation method and application thereof
A bispecific antibody, PD-L1 technology, applied in chemical instruments and methods, antibodies, specific peptides, etc., can solve problems such as reducing stromal cell TGF-β activity, stimulating immune response, tumor regression, etc., to correct immune microbes. environment, enhanced tumor suppressor activity, and high expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1 Construction of bispecific antibody YM101 binding to mouse PD-L1 and TGF-β:
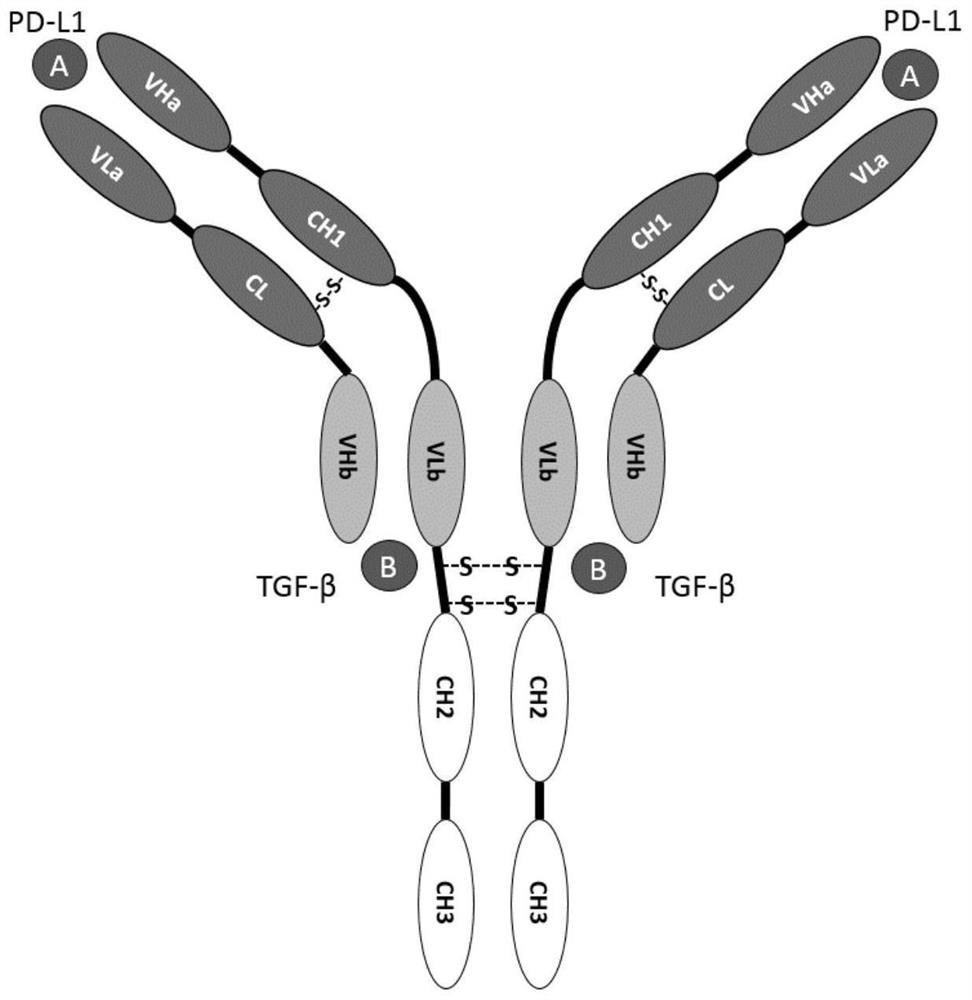
[0101] In the present invention, a bispecific antibody binding to mouse PD-L1 and TGF-β is constructed by using the commonly used molecular cloning technology. The bispecific antibody contains four polypeptide chains and can bind to two antigens. Antigen A is PD- L1, antigen B is TGF-β; specifically, the DNA sequence of the coding gene of each chain corresponding to the diabody is cloned into the pcDNA3.1(-) eukaryotic expression vector (commercially available, purchased from Invitrogen, catalog number: V79520) middle. Wherein the obtained bispecific antibody includes figure 1 The structure shown, its specific sequence information is shown in Table 2 below.
[0102] Table 2 Sequence information corresponding to the double antibody
[0103]
[0104] Combining the above table 2 and figure 1 It can be seen that the bispecific antibody constructed in the present invention includes...
Embodiment 2
[0118] Example 2 Preparation, expression and purification of bispecific antibody YM101 binding to mouse PD-L1 and TGF-β:
[0119] Plasmids were extracted according to conventional plasmid extraction methods, and used to transfect 293 cells or CHO-S cells. The transfection reagent could be Lipofectamine2000 (Thermo fisher, Cat. No. 11668019). Transfected 293 cells or CHO cells were incubated at 37°C in 5% CO 2 Suspended and shaken culture in a shaker for 7 to 10 days. The supernatant was harvested by centrifugation at 3000 xg and filtered through a 0.22 μm filter. The bispecific antibody was purified by protein A affinity chromatography and cation exchange chromatography.
[0120]The concentration of purified diabody was determined by UV absorbance at 280 nm and the corresponding extinction coefficient for each protein. The purified protein was analyzed by polyacrylamide gel electrophoresis (SDS-PAGE), and the molecular weight of the bispecific antibody was about 203KD.
[...
Embodiment 3
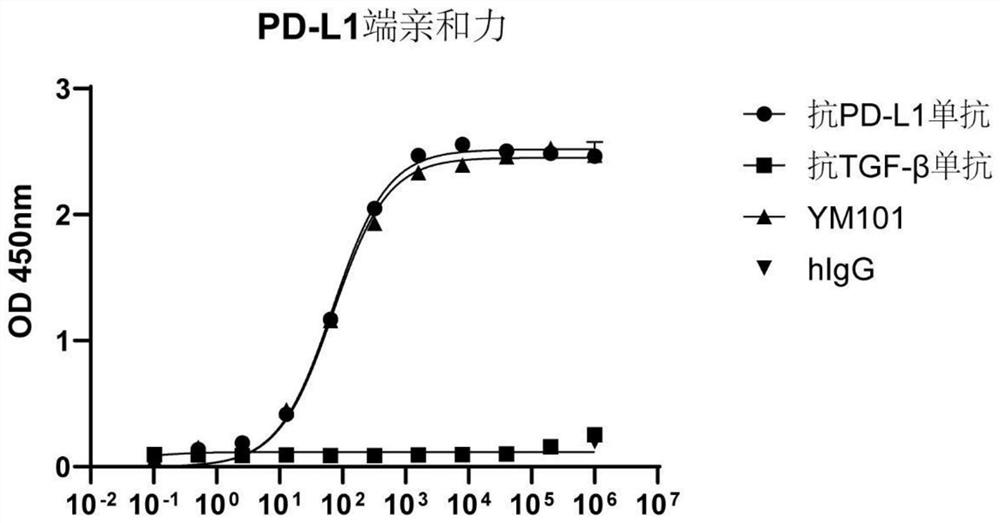
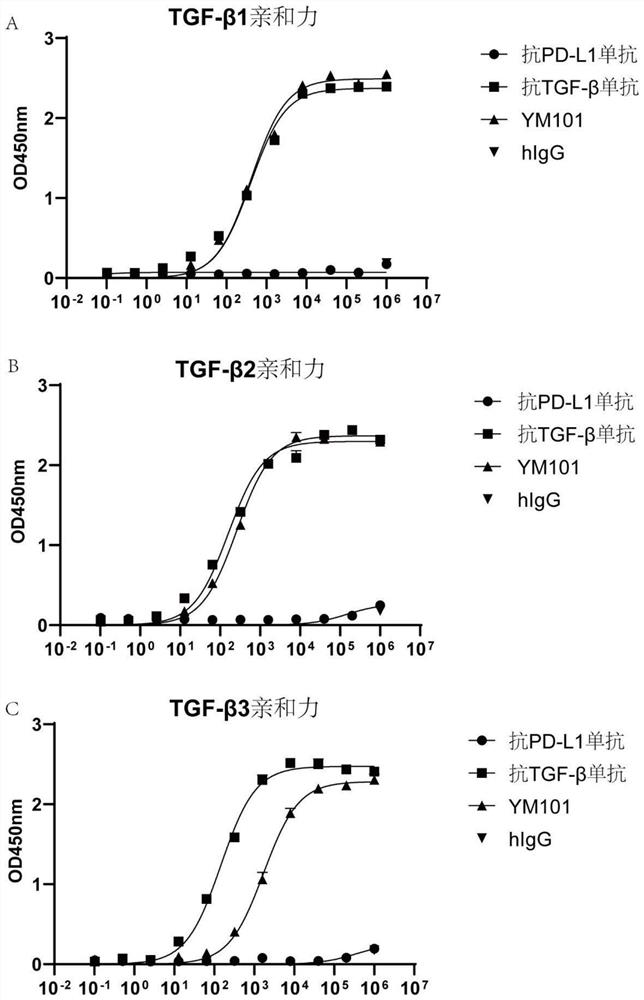
[0122] Example 3 Affinity detection of the PD-L1 end of the bispecific antibody YM101 that binds to mouse PD-L1 and TGF-β:
[0123] In this example, an enzyme-linked immunosorbent assay (ELISA) was used to detect the affinity between the bispecific antibody and PD-L1.
[0124] Among them, mouse PD-L1 (50010-M03H, Sino Biological) (200ng per well, 100ul per well) was coated on a polyacrylamide plate and kept at 4°C overnight. The plate was washed with PBS containing 0.05% Tween20, and blocked with PBS containing 3% BSA for 3 hours at 37°C. Then, add 100uL YM101 or other antibodies, and incubate at 37°C for 1 hour. Wash the plate, add 100uL HRP-labeled anti-hIgG secondary antibody (1:5000, A80-319P, Bethyl), and incubate at 37°C for 1 hour. The plate was washed, and 100 uL ELISA chromogenic substrate (555214, BD Biosciences) was added to each well. After reacting at room temperature for 15 minutes, 100 ul of 2 mol / L HCl was added to each well to stop the color development. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com