Interleukin 29 mutant protein preparation
An interleukin and protein preparation technology, applied in the field of interleukin 29 mutant protein preparations, can solve the problems of limited clinical treatment use, short in vivo half-life, poor stability, etc., to reduce degradation and aggregation, ensure non-irritant, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1IL-29
[0071] Preparation of embodiment 1 IL-29 mutant protein
[0072] 1.1 Construction of mutant protein expression engineering bacteria
[0073] The IL-29 mutant protein gene fragment SEQ ID NO:11-17 was obtained by chemical synthesis, and the above fragment was inserted into the prokaryotic expression plasmid pET-30a(+) (Novagen) through the NodeI and XhoI sites and verified by sequencing . Resulting expression plasmids for transformation assays. Transform Escherichia coli BL21(DE3) competent cells (Invitrogen) with the plasmid containing the target gene obtained above, put 50 μL of BL21 competent cells on an ice bath to melt, add the plasmid, shake gently, and place in an ice bath 30 minutes. Then heat shock in a water bath at 42°C for 30 seconds, then quickly transfer the centrifuge tube to an ice bath and let it stand for 2 minutes without shaking the centrifuge tube during this process. Add 500 μL of sterile LB medium (without antibiotics) to the centrifuge tube, mix well...
Embodiment 2
[0081] Embodiment 2 detects various indicators of the IL-29 mutant obtained
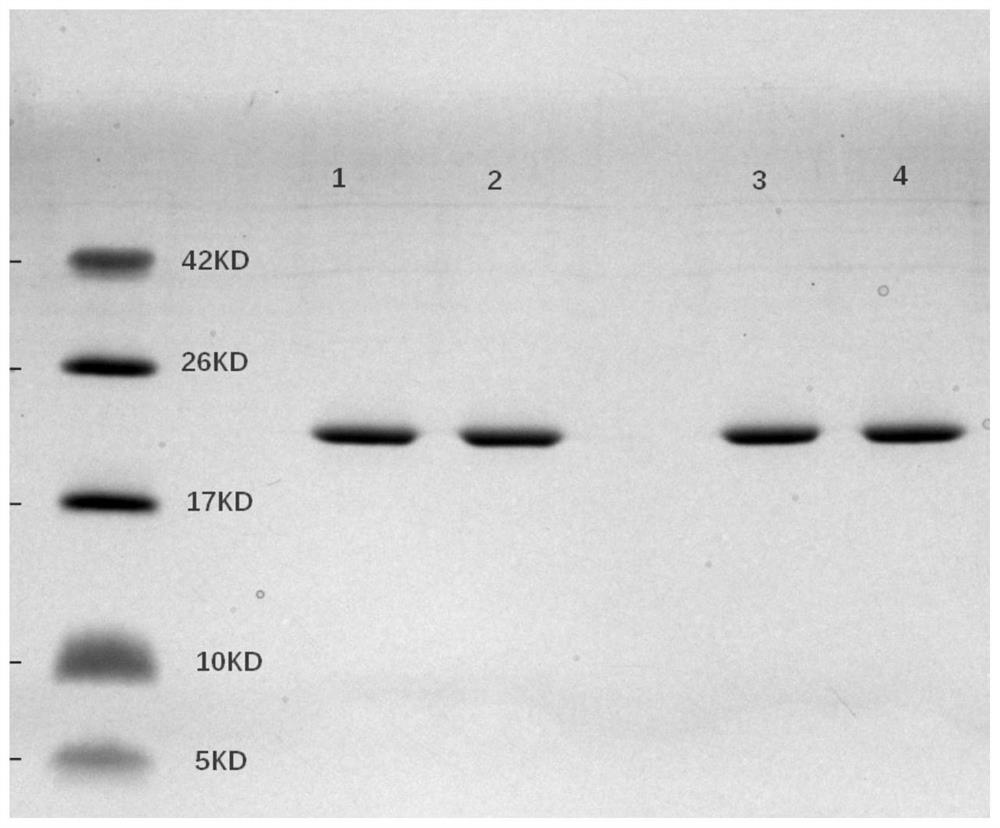
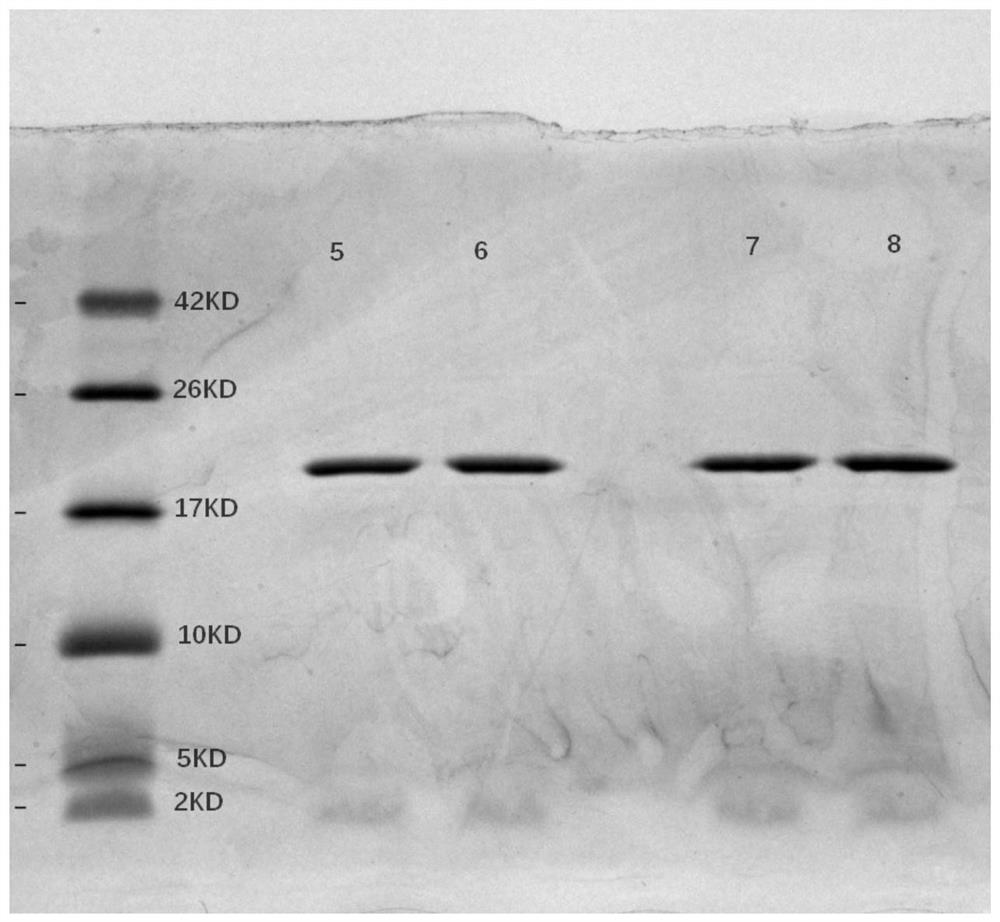
[0082] 2.1 The molecular weight and purity of IL-29 mutant obtained by SDS-PAGE electrophoresis
[0083] Using the SDS-PAGE electrophoresis loading buffer, in the case of adding mercaptoethanol, the Marker and 10 μg of the protein obtained above were loaded separately, and electrophoresis was performed. The electrophoresis condition was 200V constant voltage for 45 minutes. Stained with Coomassie Brilliant Blue G-25 to detect protein molecular weight and purity, the results are shown in figure 1 and 2 shown.
[0084] Depend on figure 1 and 2 It can be seen that the molecular weights of the IL29 mutant protein and the reference substance are 20 kDa respectively, indicating that the obtained target protein is correct, with only one band without other impurities, and the purity can reach 100%.
[0085] 2.2 In vitro RP-HPLC purity of IL-29 mutants detected by chromatography
[0086] 2.2.1 Reversed ...
Embodiment 3I
[0102] The 50 ℃ stability detection result of embodiment 3 IL-29 mutants
[0103] The stability of the protein in this application is mainly characterized by reversed-phase HPLC purity.
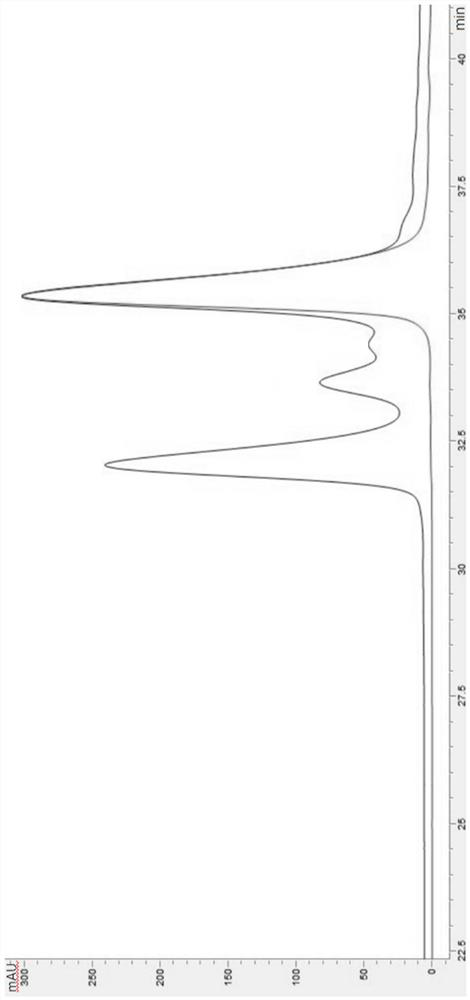
[0104] Under the conditions of 50°C ± 2°C / 75% relative humidity ± 5% relative humidity, samples were taken according to Table 4, and the reverse phase of each test product of IL-29 mutant was carried out in the same way as 2.2.1 in Example 2 For HPLC purity determination, the same method as 2.3 in Example 2 was used to measure the biological activity of each test product of the IL-29 mutant, and the results are shown in Table 5. Simultaneously compare the chromatograms of the purity values of 0 days and 14 days in Table 5, see for details Figure 3-10 .
[0105] Table 4 Stability verification scheme of IL-29 mutants at 50°C
[0106]
[0107] Table 5 Determination results of RP-HPLC purity of IL-29 mutants at 50°C
[0108]
[0109]
[0110] From Table 5 above and Figure 3-10 T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass volume concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com