Preparation method of nicotine and intermediate thereof
A technology of intermediates and nicotine, which is applied in the field of preparation of nicotine and its intermediates, can solve the problems of low conversion rate of intermediates and harsh reaction conditions, and achieve the effects of high conversion rate, easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The second aspect of the present invention relates to the preparation method of the above-mentioned intermediate, comprising the following steps:
[0066] S110: reacting the compound shown in formula (II-1) and the compound shown in formula (II-2) to prepare the compound shown in formula (II-3).
[0067]
[0068] R 1 As defined in the first aspect of the present invention, details are not repeated here.
[0069] The compound represented by formula (II-1) (ie, 3-pyridinecarbaldehyde) and the compound represented by formula (II-2) can be commercially available raw materials, or can be prepared by existing methods.
[0070] In one embodiment, the compound shown in formula (II-2) is tert-butylsulfinamide (i.e. R 1 for tert-butyl).
[0071] In one embodiment, step S110 includes the following steps:
[0072] S111: Mix the compound represented by the formula (II-1), the compound represented by the formula (II-2), the base and the solvent, and react at a temperature of 2...
Embodiment 1
[0143]
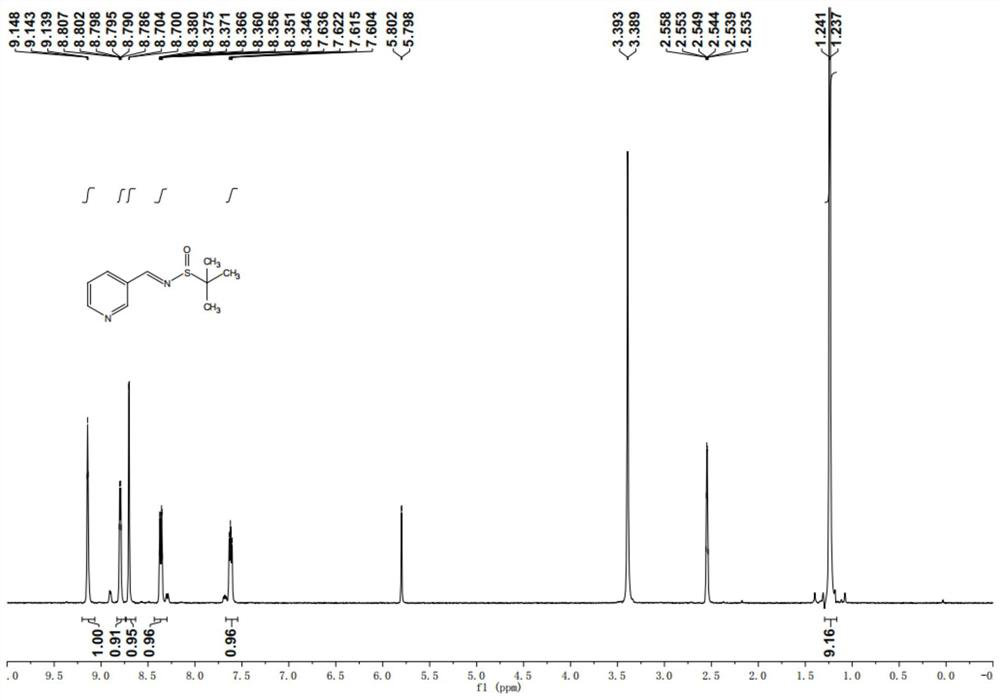
[0144] Take 100g of 3-pyridinecarbaldehyde, 100.5g of tert-butylsulfinamide, and 180.5g of cesium carbonate, dissolve them in 700ml of dichloromethane, stir and react at 25°C (room temperature) for 4h, and check the completion of the reaction by LC-MS. The reaction is complete Afterwards, the solid impurities were removed by filtration, and the dichloromethane solvent was recovered by distillation under reduced pressure to obtain 190.5 g of 2-methyl-N-(pyridine-3-methylene)propane-2-sulfinamide, with a yield of 97%. figure 1 .
Embodiment 2
[0146]
[0147] Take 95g of magnesium chips, disperse them with 200ml of tetrahydrofuran, and put them into the reaction flask, under nitrogen protection, dissolve 2-(2-bromoethyl)-1,3-dioxane with 300ml of tetrahydrofuran, and then put it in an ice bath ( -15°C) was added into the reaction flask in 6 batches within 1 hour, and after the exothermic end, the reaction temperature was raised to 50°C for 4 hours to initiate the Grignard reagent.
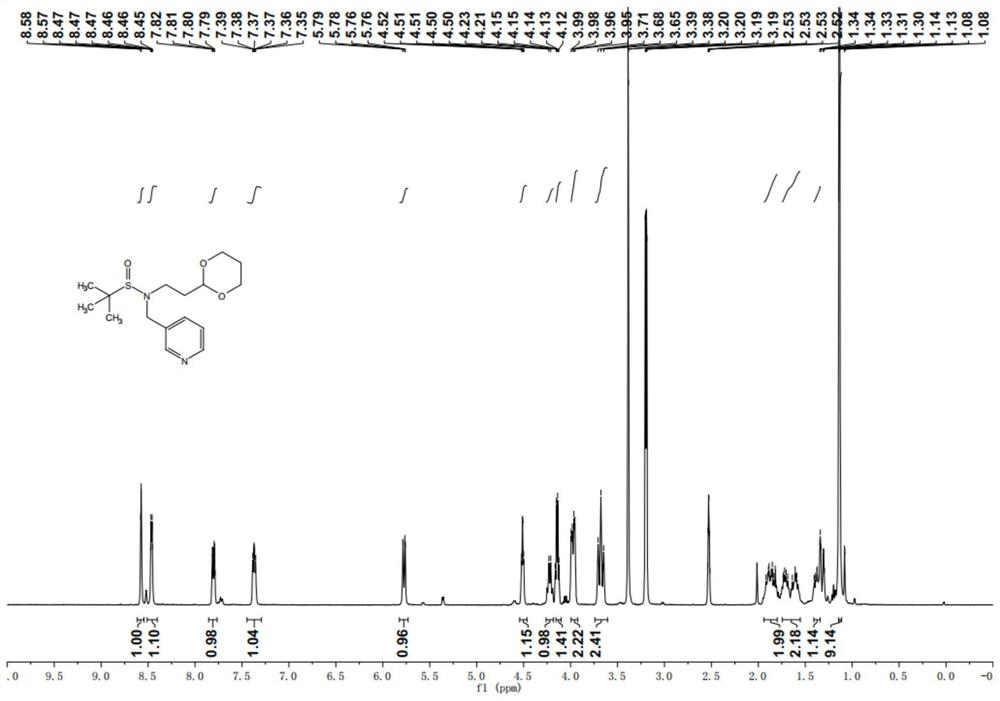
[0148] Take 190g of the product 2-methyl-N-(pyridine-3-methylene)propane-2-sulfinamide in Example 1, dissolve it in 500ml of tetrahydrofuran, and then divide it in an ice bath (-15°C) within 1h. Add to the reaction flask five times, react at 25°C (room temperature) for 5h, check the completion of the reaction by LC-MS, after the reaction is complete, quench the reaction with saturated ammonium chloride aqueous solution, filter the solid impurities, concentrate the water phase volume to 800ml, and use 1200ml of ethyl acetate was extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com