Preparation method of scopolamine butylbromide injection
A technology of scopolamine and injection, applied in the field of scopolamine butylbromide injection and preparation thereof, can solve the problems of temperature, light sensitivity, less research on injection, complicated preparation process, etc., and achieves stable quality, low adverse reaction rate, Component simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
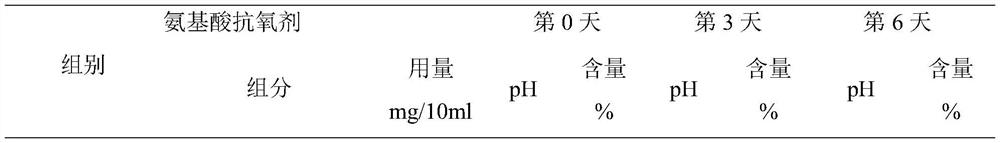
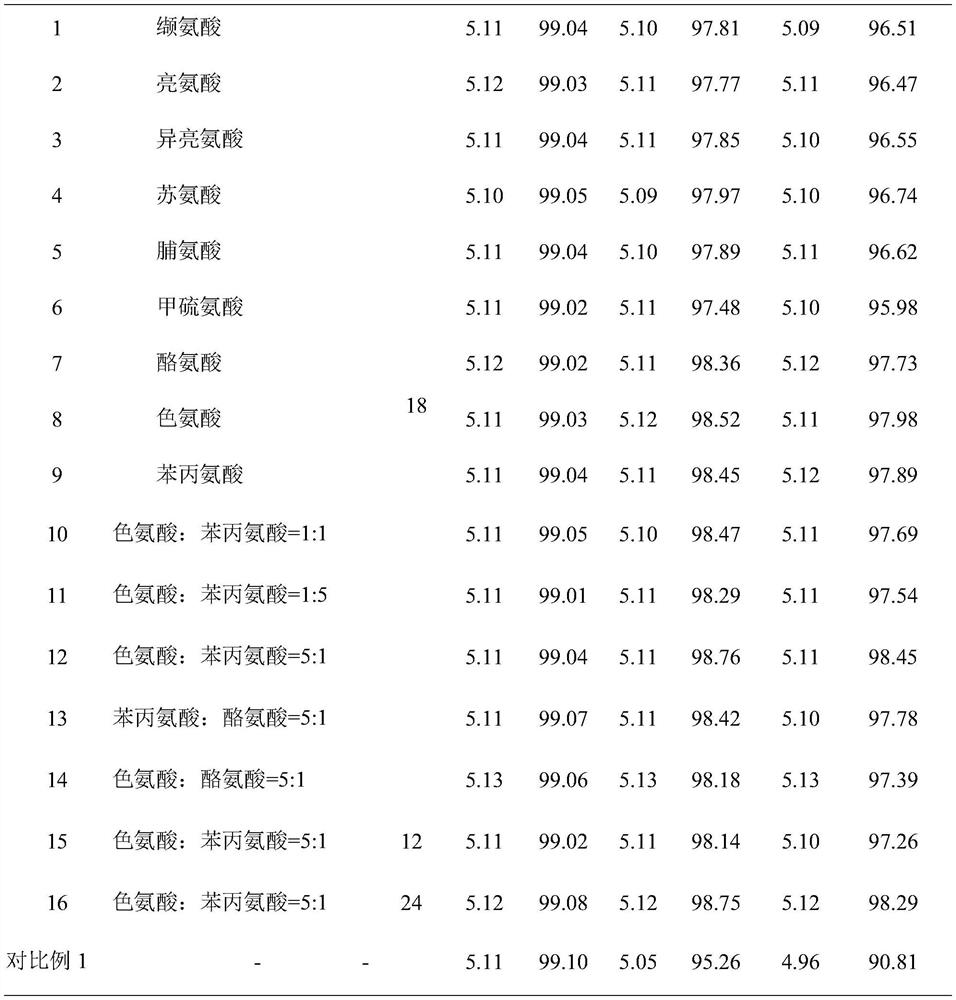
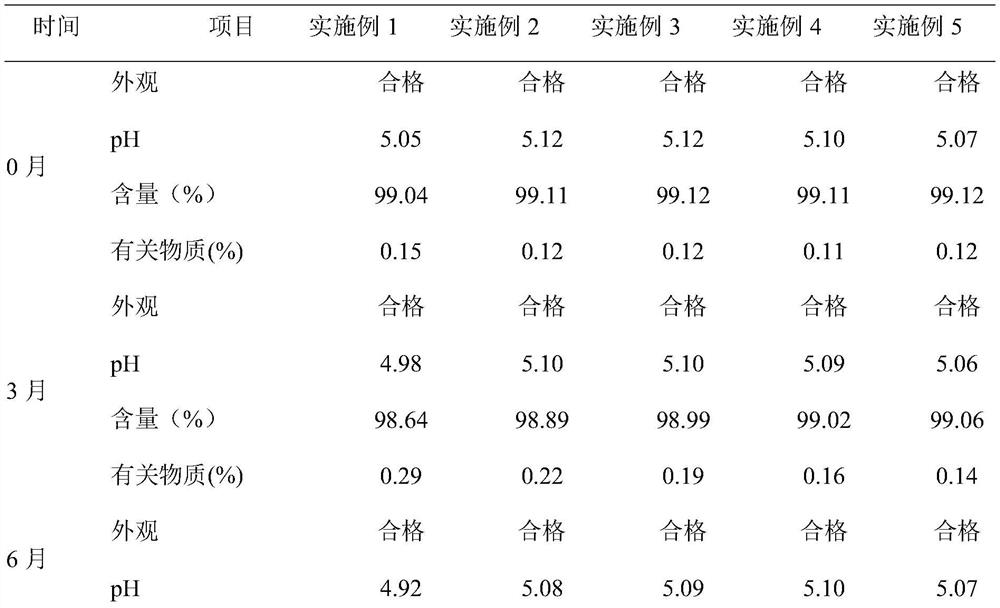
[0026] A scopolamine butylbromide injection, containing scopolamine butylbromide 200mg, glutathione 4.2mg, water for injection added to 10ml, pH value is 5.05.
[0027]The preparation method comprises: (1) taking 70% of the prescribed amount of water for injection, and adjusting the pH value to 5.1 with 0.1M hydrochloric acid; (2) adding the prescribed amount of scopolamine butylbromide and stirring at 150 rpm for 30 minutes; (3) adding the prescribed amount of glutathione The peptide and the remaining water for injection were stirred for 25 minutes, filtered through a 0.22 μm microporous membrane, filled, filled with nitrogen protection during the entire preparation and filling process, and sterilized at 121 ° C for 12 minutes to obtain the product.
Embodiment 2
[0029] A scopolamine butyl bromide injection, containing 200 mg scopolamine butyl bromide, 15 mg tryptophan, 3 mg phenylalanine, 19 mg disodium hydrogen phosphate, 7 mg citric acid, added to 10 ml of water for injection, the pH value is 4.92.
[0030] The preparation method comprises: (1) taking 60% of the prescribed amount of water for injection, adding disodium hydrogen phosphate and citric acid in the prescribed amount, stirring at 180 rpm for 12 minutes to completely dissolve it; (2) adding the prescribed amount of scopolamine butylbromide, and stirring at 120 rpm 30min; (3) Add the prescribed amount of tryptophan, phenylalanine and the remaining water for injection, control the pH to 4.9, stir for 20min, filter through a 0.22μm microporous membrane, fill, and fill in the whole preparation and filling process Nitrogen protection, sterilized at 121°C for 12 minutes, ready to use.
Embodiment 3
[0032] A scopolamine butylbromide injection, containing scopolamine butylbromide 200mg, tryptophan 15mg, phenylalanine 3mg, disodium hydrogen phosphate 19mg, citric acid 7mg and water for injection is added to 10ml, pH value is 4.9.
[0033] The water for injection of scopolamine butylbromide injection is pretreated, and other operations are the same as in Example 2. The pretreatment is as follows: the stirred tank is pre-filled with nitrogen, heated to 62°C after adding water for injection, vacuumed to 0.035Mpa, and stirred under reduced pressure for 30 minutes; slowly lowered to 4°C at a speed of 8°C / h, and passed to the stirred tank Inject nitrogen gas to 0.25Mpa, pressurize, stir for 30min, and place at room temperature and pressure for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com