A kind of coumarin derivative CTT and its synthesis method and application
A technology of coumarin derivatives and synthesis methods, applied in chemical instruments and methods, fluorescence/phosphorescence, instruments, etc., to achieve high sensitivity and simple detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
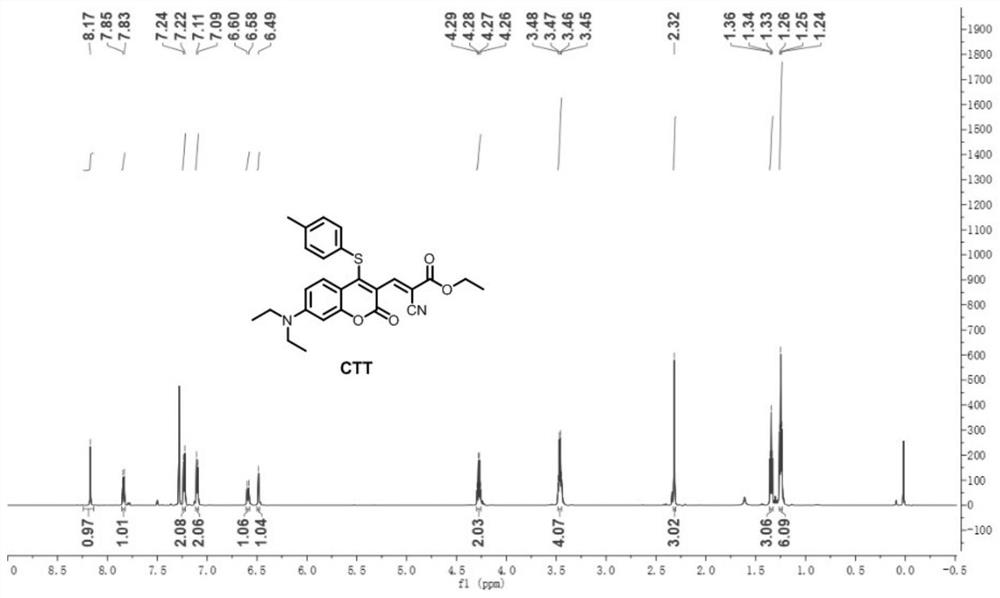
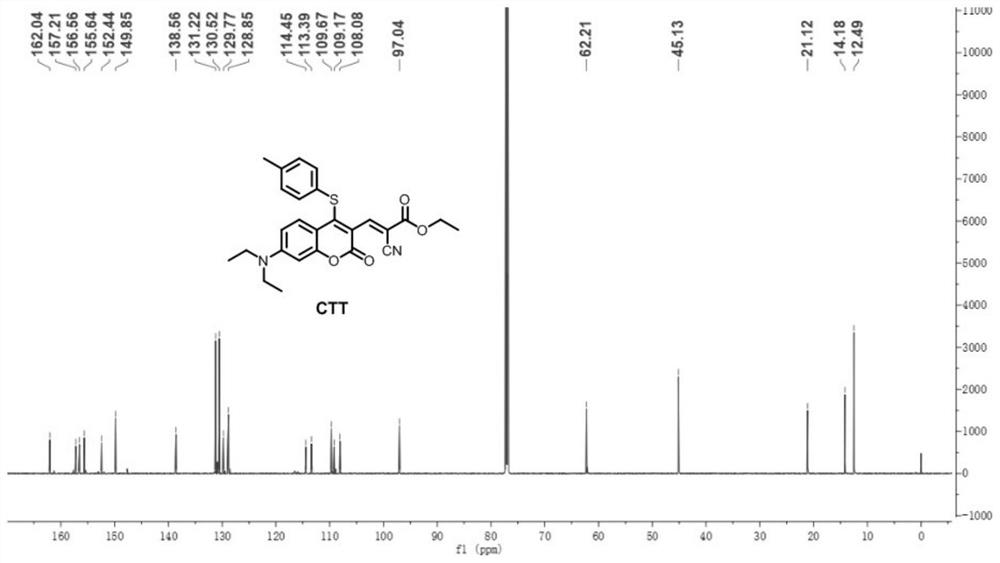
[0033] Preparation and characterization of CTT
[0034] Synthetic route of CTT:
[0035]
[0036] The synthetic method of CTT, the steps are:
[0037] (1) 11.5 mL of POCl was slowly added to a mixture of 11.0 g of malonic acid and 19.95 g of phenol at 0°C 3 . The mixture was heated at 115°C until the strong release of HCl ceased (about 1.5 hours). The upper layer was poured into 150 mL of water, extracted three times with EtOAc, and then routinely worked up to give diphenyl malonate (compound 1, 20.6 g, 98% yield) as a pale brown oil, which was pure enough to be used without further purification Next step.
[0038] (2) To a solution of 12.8 g of Compound 1 in toluene (50 mL) was added 8.25 g of 3-N,N-diethylaminophenol. The reaction mixture was refluxed for 7 hours. After the reaction was completed, the filter cake was filtered and washed with hexane. The product was dried under vacuum to give a pale yellow solid (compound 2, 8.7 g, 74% yield).
[0039] (3) Under ni...
Embodiment 2
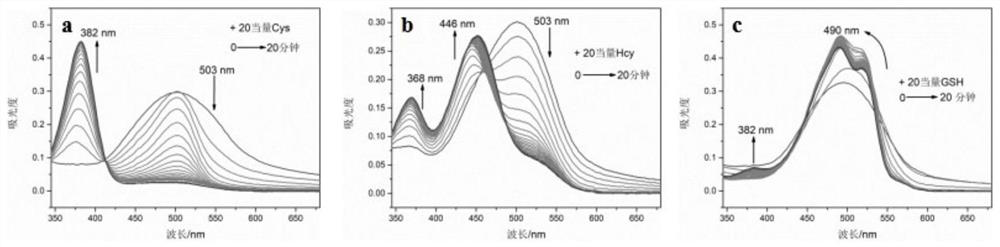
[0043] Take 1600 μL of PBS buffer solution and 400 μL of DMSO in a cuvette, add 10 μL of CTT DMSO solution to it, and prepare three parts in total, add 200 μM of Cys, Hcy and GSH respectively, and monitor their changes with time by ultraviolet spectroscopy. The results show that : For Cys, the absorption at 503nm decreases rapidly, while a new absorption appears at 382nm and increases rapidly; for Hcy, while the original absorption peak at 503nm gradually disappears, new absorption appears at 368nm and 446nm and continues to increase; for GSH , the absorption at 503nm shifted to 490nm and increased slowly, while a new absorption peak appeared at 382nm with a small enhancement (see image 3 ).
Embodiment 3
[0045] Dissolve 1600 μL of PBS buffer solution and 400 μL of DMSO in the cuvette, and then add 10 μL of CTT DMSO solution to it to prepare three parts. fluorescence to monitor their spectral changes. After the reaction of Cys with the probe CTT, the orange-red fluorescence at 582nm was rapidly quenched, and a strong blue fluorescence emission appeared at 466nm; after the reaction of CTT with Hcy, the fluorescence at 582nm also disappeared, while strong blue fluorescence appeared at 456nm and 548nm, respectively. and yellow fluorescence; for GSH, the probe CTT original emission at 582nm was blue-shifted to 557nm, changing from orange-red to yellow emission (see Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com