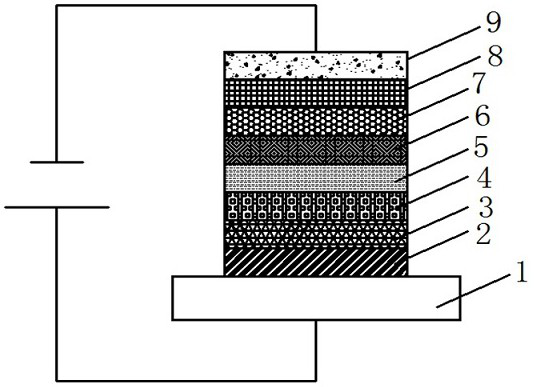
Organic compound and organic electroluminescent device
An organic compound and electromechanical technology, applied in the field of organic compounds and organic electroluminescent devices, can solve problems such as backwardness and insufficient development of organic electroluminescent materials, so as to avoid reverse transfer, improve chemical stability and thermal stability, The effect of improving efficiency and life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
[0075] The synthetic method of compound 2 is as follows:
[0076]
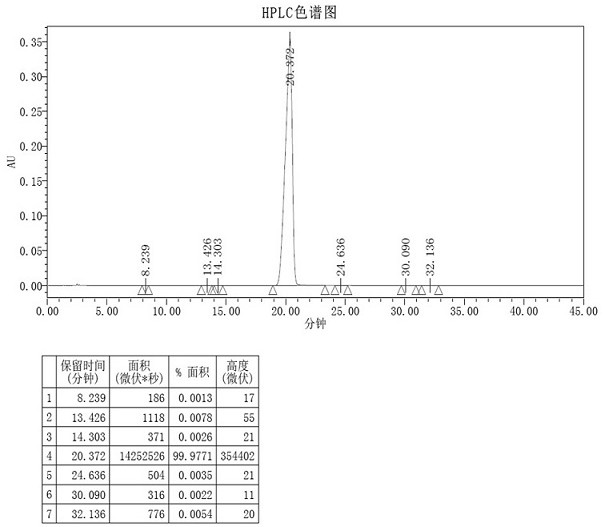
[0077] Under nitrogen protection, compound 1-a (1.1eq, 9.5g, 375.28g / mol, 25.41mmol) and compound 1-b (1eq, 10g, 432.90g / mol, 23.10mmol) were dissolved in 200mL toluene, and acetic acid was added Palladium (0.26g, 224.51g / mol, 1.16mmol), X-phos (0.26g, 476.72g / mol, 1.16mmol), potassium carbonate (9.58g, 138.21g / mol, 69.3mmol), then add 100mL ethanol and 50mL of water was stirred overnight at 82°C, and the reaction progress was monitored by HPLC.
[0078] After the complete reaction of compound 1-b was monitored by HPLC, the reaction was stopped, the reaction solution was cooled to room temperature, 60 mL of water was added, stirred for 20 min, and the filter cake was obtained by suction filtration. The filter cake was rinsed twice with water and ethanol and then dried in vacuum at 80°C for 6 hours. Add the dried filter cake to a 250mL three-necked flask, add 100mL of o-dichlorobenzene, and heat...
Embodiment 2
[0080]
[0081] The synthetic method of compound 5 is as follows:
[0082]
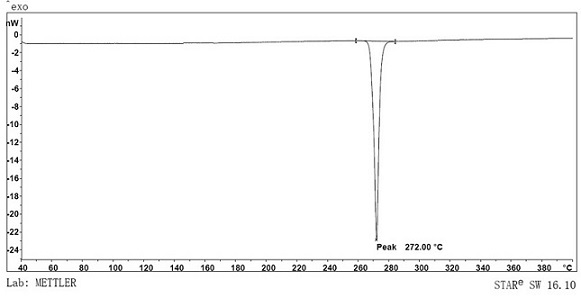
[0083] The preparation method is basically the same as in Example 1, the difference is that compound 1-a is replaced by compound 2-a to obtain the final target product compound 5 (9.2g, yield 61.3%), ESI-MS (m / z) (M+ ): theoretical value 645.76, measured value 646.15, elemental analysis results (molecular formula C45H23D5N4O): theoretical value C, 83.70; H, 5.15; N, 8.68; O, 2.48; measured value C, 83.71; H, 5.14; N, 8.62; O, 2.53.
Embodiment 3
[0085]
[0086] The synthetic method of compound 6 is as follows:
[0087]
[0088] The preparation method is basically the same as in Example 1, the difference is that compound 1-a is replaced by compound 3-a, compound 1-b is replaced by compound 3-b, and the final target product compound 6 (9.7g, yield 65% ), ESI-MS (m / z) (M+): theoretical value 645.76, measured value 646.02, elemental analysis results (molecular formula C45H23D5N4O): theoretical value C, 83.70; H, 5.15; N, 8.68; O, 2.48; measured value C, 83.68; H, 5.17; N, 8.70; O, 2.45.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com