Circularly polarized light-emitting organic micro-nano crystal material as well as preparation method and application thereof
A crystalline material and circular polarization technology, applied in the field of circularly polarized light-emitting organic micro-nano crystal materials and their preparation, can solve the problems of complex molecular structure, long and tedious preparation process, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
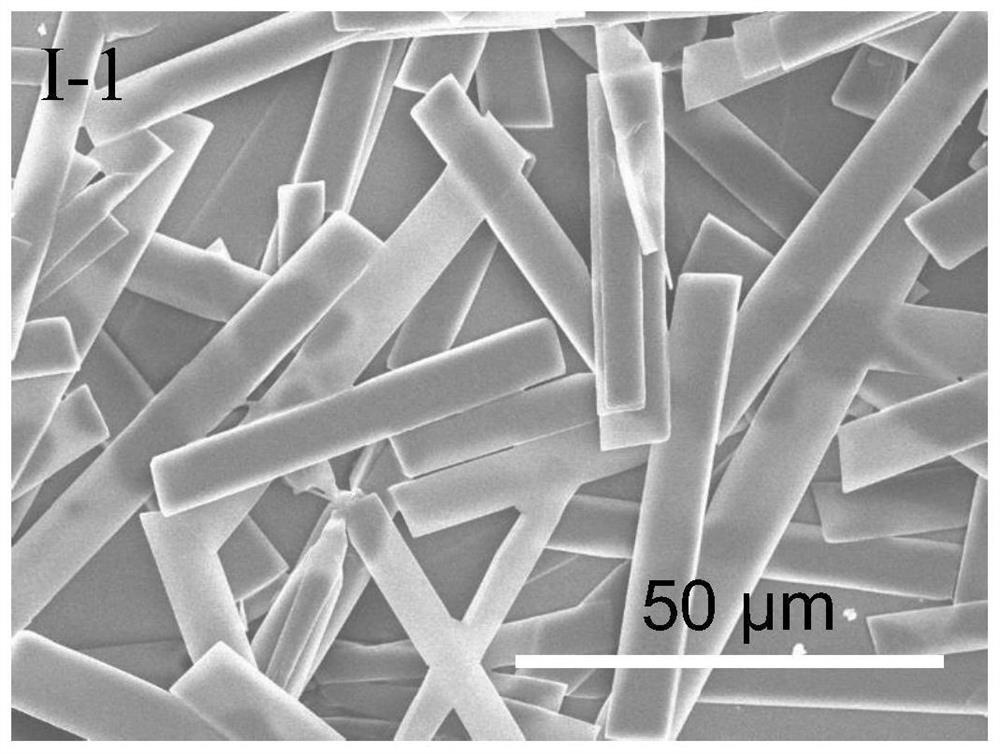
[0102] This embodiment provides an ionic protonated pyridinium salt compound as shown in formula I-1 and a preparation method thereof.
[0103]
[0104] The preparation method of the ionic protonated pyridinium salt compound comprises the following steps:
[0105] (1) Preparation of 2,2'-bis(2-pyridine)-1,1'-biphenyl:
[0106] The synthesis of 2,2'-bis(2-pyridine)-1,1'-biphenyl refers to the reaction formula (1-1) Guo X, Wang G, Li C-J.Ruthenium-Catalyzed Oxidative Homo-Coupling of 2-Arylpyridines[ J]. Adv. Syn. Cat. 2009, 351:2071-2074.
[0107]
[0108] (2) Preparation of ionic protonated pyridinium salt complexes as shown in formula I-1a:
[0109]
[0110] A solution of 2,2'-bis(2-pyridine)-1,1'-biphenyl (6.9 mg, 0.022 mmol) in tetrahydrofuran (1 mL) and levocamphorsulfonic acid (L-(-)-CSA) (10.2 mg , 0.044mmol) tetrahydrofuran solution (2mL) uniformly mixed, 70W water bath ultrasonic 2min, room temperature for 10min, filtered the white precipitated solid to obt...
Embodiment 2
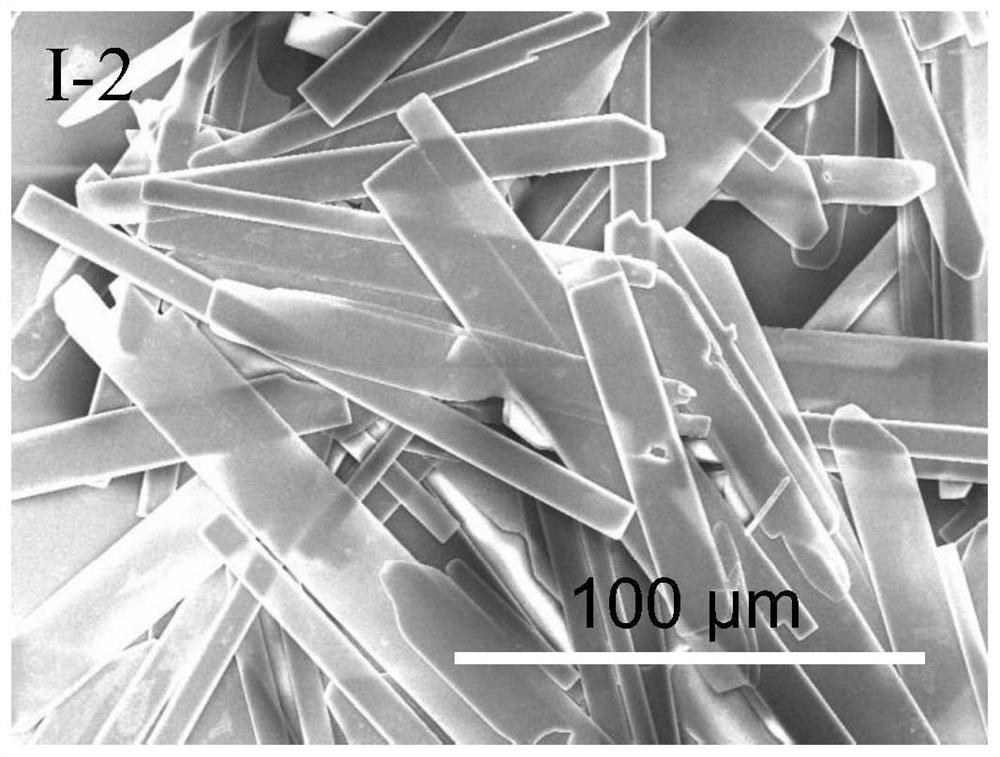
[0122] This embodiment provides an ionic protonated pyridinium salt compound as shown in formula I-2 and a preparation method thereof.
[0123]
[0124] Its preparation method comprises the following steps:
[0125] (1) Preparation of 2,2'-bis(4-bromo-2-pyridine)-1,1'-biphenyl;
[0126] Dimer of p-cymene dichloride ruthenium (76.5mg, 0.13mmol), 4-bromo-2-phenylpyridine (1170mg, 5.0mmol), ferric chloride (649mg, 4.0mmol) and 15mL of chlorobenzene Added to a 100mL oven-dried reaction vessel, stirred at 120°C for 16h. After cooling to room temperature, 12mL Et 3 N and 12mL CH 2 Cl 2 Added to the reaction system, and continued to stir at room temperature for 0.5h. The resulting mixture was treated with CH 2 Cl 2 Diluted short silica gel column was filtered, the filtrate was concentrated, and the residue was purified by column chromatography (SiO 2, PE / EA=5:1, v / v), 550 mg of white solid was obtained, the yield was 64%.
[0127] The main reaction path is shown in reacti...
Embodiment 3
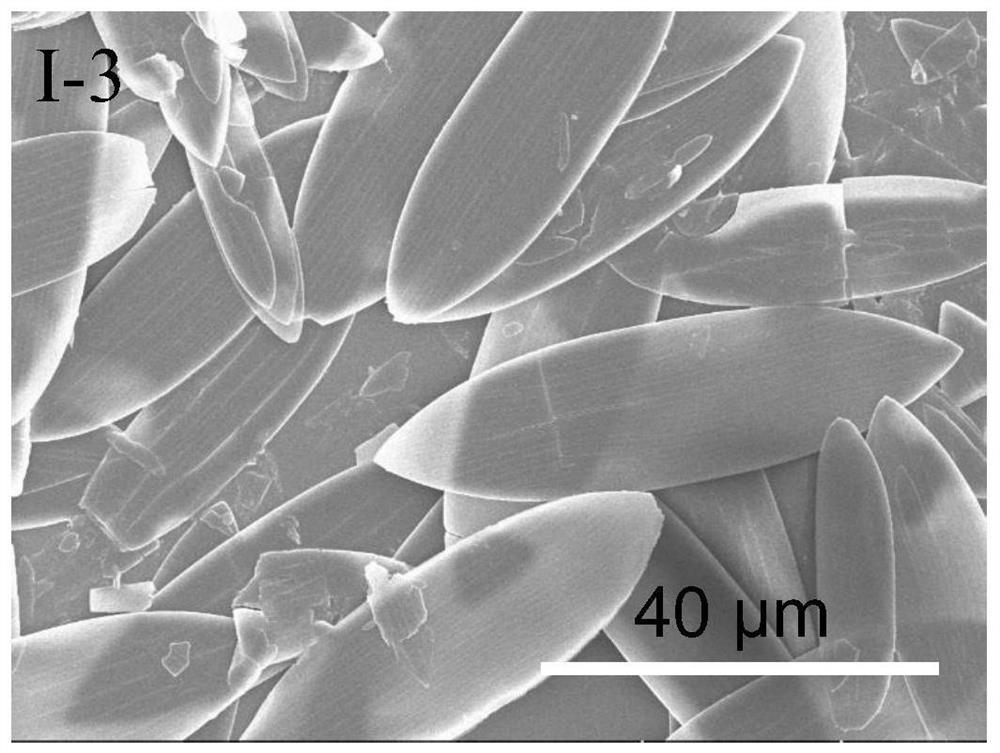
[0153] This embodiment provides an ionic protonated pyridinium salt compound as shown in formula I-3 and a preparation method thereof.
[0154]
[0155] The preparation method of this compound comprises the following steps:
[0156] (1) Preparation of 2,2'-bis(4-((E)-4-methoxystyrene)-2-pyridine)-1,1'-biphenyl:
[0157] in N 2 Under protection, 2,2'-bis(4-bromo-2-pyridine)-1,1'-biphenyl (322mg, 0.69mmol), K 2 CO 3 (193mg, 1.4mmol) and Pd(OAc) 2 (18mg, 0.08mmol) was added to an oven-dried 100mL round bottom flask, and 1-methoxy-4-vinylbenzene (375μL, 2.8mmol) and DMF (8mL) were added with a syringe, and nitrogen was bubbled at room temperature to remove oxygen for 20min After that, add PPh 3 Ligand (56mg, 0.21mmol), the reaction system was sealed and placed in an oil bath preheated to 140°C, and stirred at this temperature for 48h. After cooling to room temperature, the reaction mixture was poured into water (10 mL) and washed with CH 2 Cl 2 (3 x 20 mL) extraction. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com