Preparation method and application of nickel hydroxide/nickel electrode
A nickel hydroxide and nickel electrode technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve problems such as unsatisfactory catalytic activity, and achieve the effects of excellent catalytic performance, short preparation time and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: α-Ni(OH) 2 Preparation of / NF self-supporting electrodes
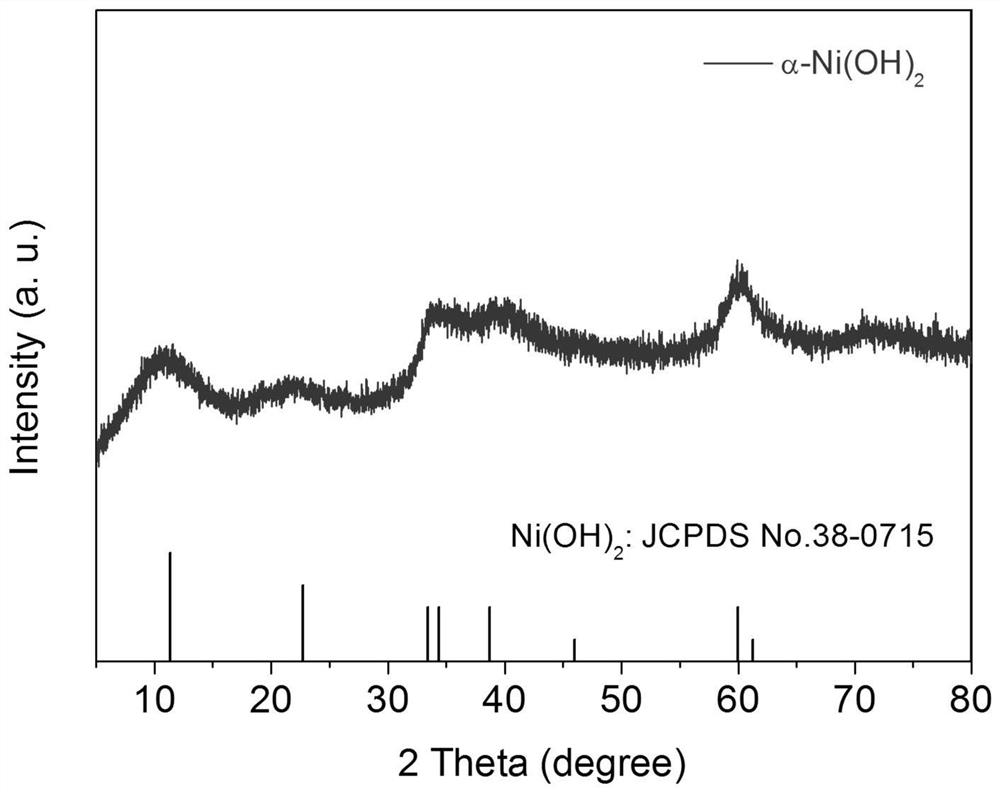
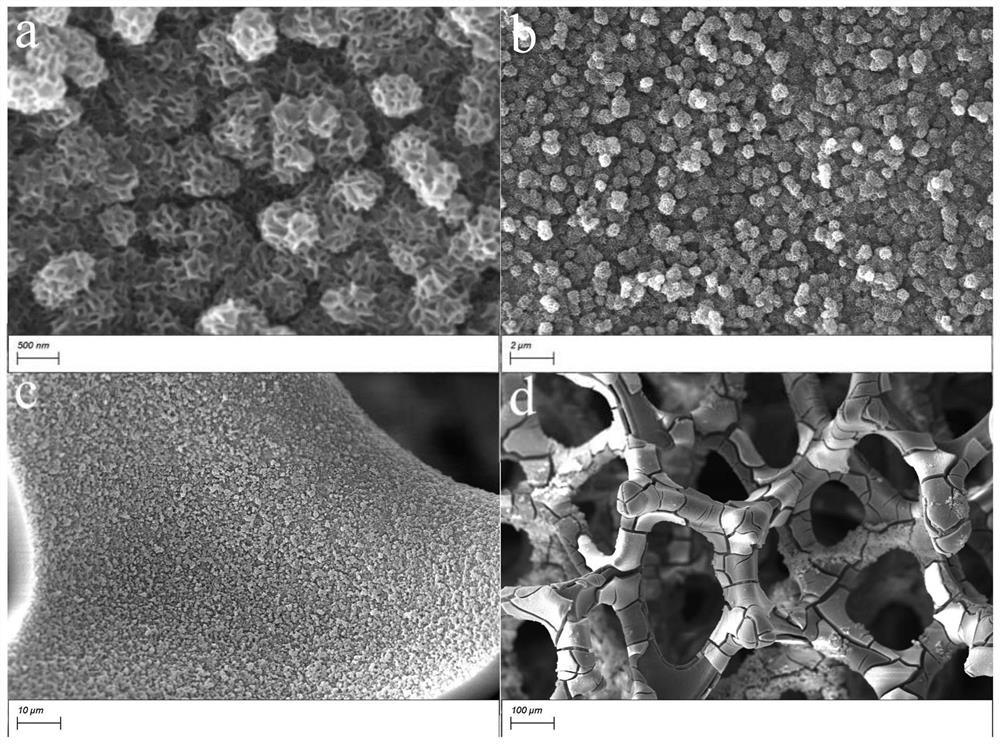
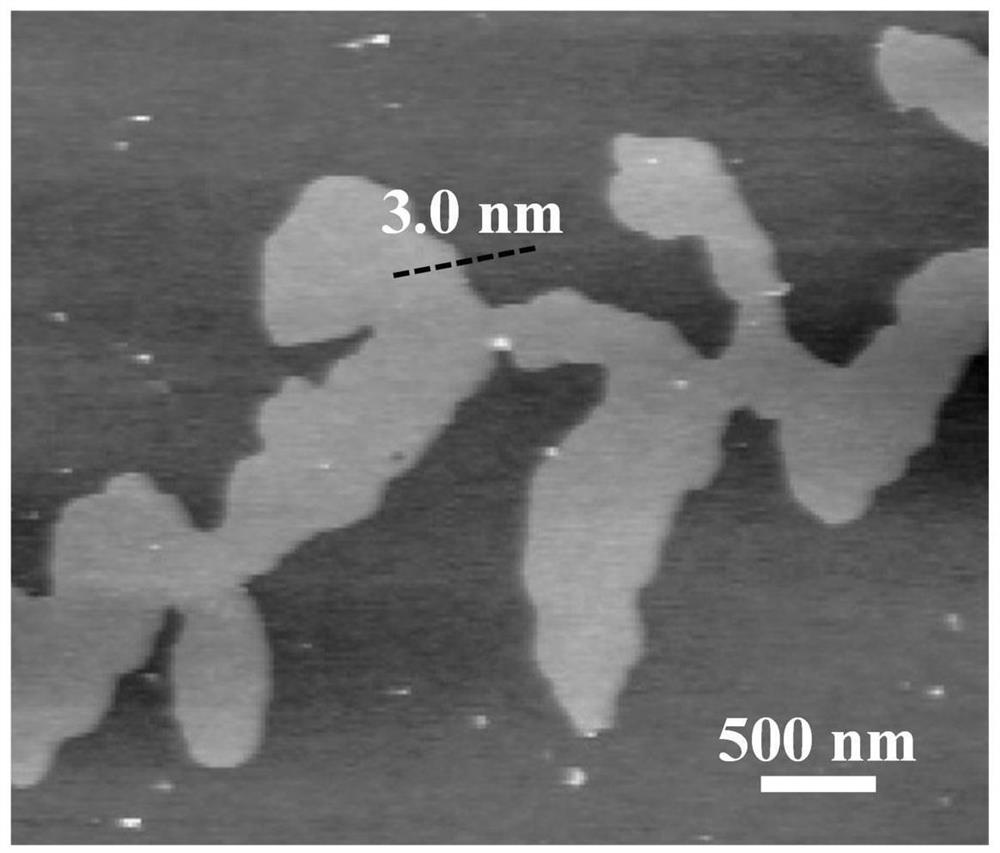
[0033] Nickel foam (NF, thickness: 1.7mm) was ultrasonically cleaned in ethanol, 3.0M hydrochloric acid solution and ultrapure water for 10 minutes; then, the prepared 0.2M Ni(NO 3 ) 2 . 6H 2Pour the O aqueous solution into the electrolytic cell; then, insert the cleaned NF working electrode (immersion area of 1cm×3cm), Pt counter electrode and Ag / AgCl reference electrode into the above electrolyte; finally, connect the electrochemical workstation, α-Ni(OH) can be prepared by deposition for 300s at the cathodic deposition potential of -2.0V vs Ag / AgCl 2 / NF self-supporting electrodes. The X-ray diffraction pattern of the product is shown in figure 1 ; Scanning Electron Microscopy, see Figure 12 ; Atomic force microscope image see image 3 ; transmission electron microscope image see Figure 4 .
Embodiment 2
[0034] Embodiment 2: the α-Ni(OH) that embodiment 1 gains 2 / NF electrocatalytic OER performance test
[0035] The α-Ni(OH) of embodiment 1 gained 2 The electrocatalytic OER performance test of / NF was carried out on a CHI760E electrochemical workstation. The electrolyte is 1.0M KOH aqueous solution. Using α-Ni(OH) 2 / NF, Ag / AgCl, and Pt plates were used as working, reference, and counter electrodes, respectively. Figure 5 The linear sweep voltammetry curve shown is obtained at a sweep rate of 1.0mV / s, and it can be seen in the figure that α-Ni(OH) 2 / NF driven 100 and 250mA cm -2 The required overpotentials for the current densities are 240 and 290 mV, respectively. Figure 6 The Tafel plot shown is from Figure 5 Calculated, it can be seen that α-Ni(OH) 2 The Tafel slope of / NF is 108mV·dec -1 . Figure 7 The potential diagram required for the test under different high current densities shown is the α-Ni(OH) 2 / NF drive 500-1000mA cm -2 The current density is fi...
Embodiment 3
[0036] Embodiment 3: the α-Ni(OH) that embodiment 1 gains 2 / NF electrochemical specific surface area test
[0037] To determine the electrochemical surface area (ECSA), cyclic voltammetry (CV) measurements were used to explore the electrochemical double layer capacitance (C dl ). CV in non-Faraday range (0.93-1.03V vs RHE) with sweep rates of 60, 80, 100, 120, 140 and 160mV s -1 . A linearity plot was obtained by plotting current density versus scan rate at 0.98V vs RHE. C dl is half the slope of the linear graph and is used to represent ECSA. The electrochemical specific surface area diagram is shown in Figure 9 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com