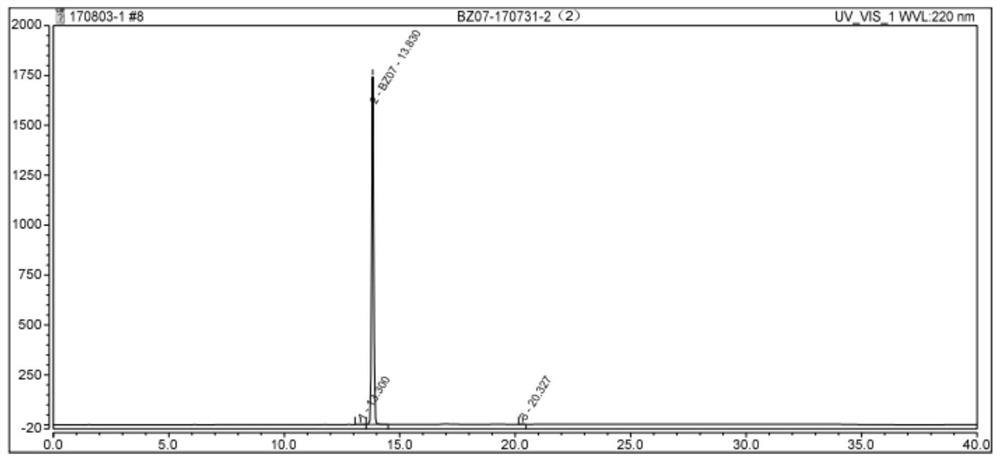
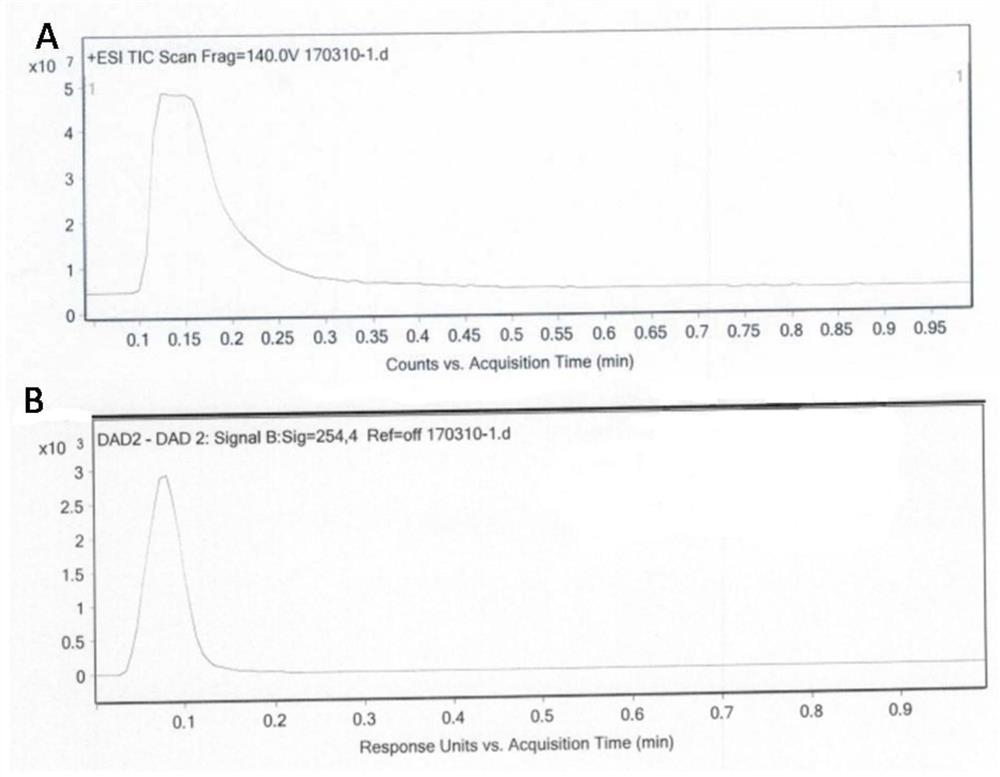
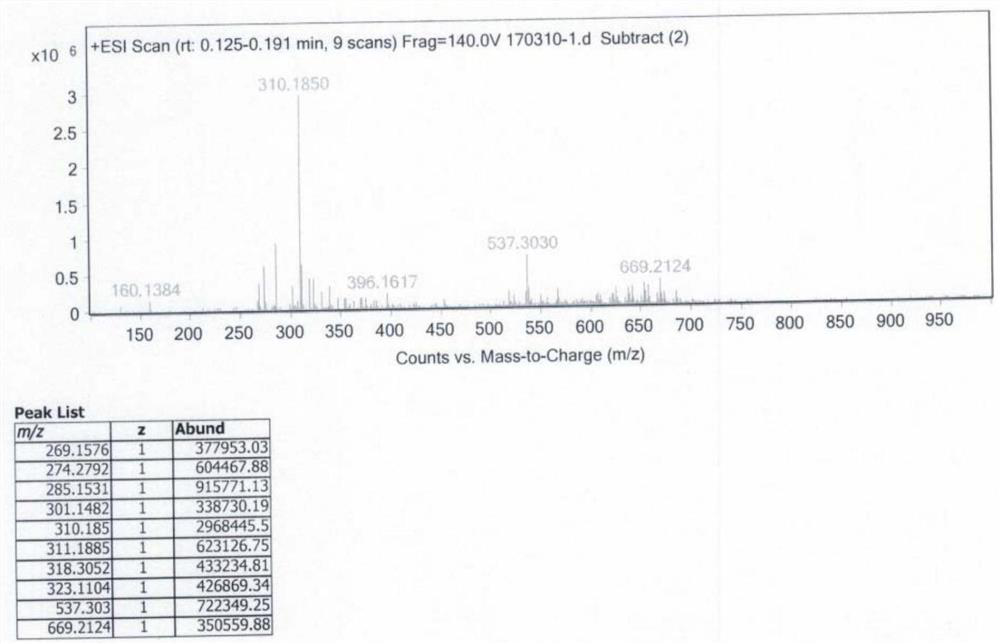
Benvitimod impurity as well as preparation method and application thereof
A technology of phenylene moder and impurity, which is applied in the field of drug synthesis to achieve the effect of strengthening control, ensuring safety and effectiveness, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] As an embodiment of the preparation method of the phenylene Moder impurity described in the present invention, the preparation method of the Benzene Moder impurity described in this embodiment comprises the following steps:
[0039] (1) Take 1 kg of E-1,3-dimethoxy-2-isopropyl-5-(2-styryl)-benzene and 2.0 kg of pyridine hydrochloride, put them in a reaction vessel, heat and keep Stir the reaction at 200°C, monitor by HPLC, stop the reaction until the reaction of E-1,3-dimethoxy-2-isopropyl-5-(2-styryl)-benzene is complete;
[0040] (2) Take 13.0L of ethyl acetate, 9.0L of drinking water and 0.28L of hydrochloric acid, put them in the extraction container, stir, transfer the reaction solution in step (2) to the extraction container while it is hot, stir, let it stand, and discard the lower layer Water phase; add 8.5L drinking water and 0.14L hydrochloric acid, stir, let stand, discard the lower aqueous phase; add 4.0L drinking water, stir, let stand, discard the lower aq...
Embodiment 2
[0052] As an embodiment of the preparation method of the phenylene Moder impurity described in the present invention, the preparation method of the Benzene Moder impurity described in this embodiment comprises the following steps:
[0053] (1) Take E-1,3-dimethoxy-2-isopropyl-5-(2-styryl)-benzene Akg and pyridine hydrochloride 2.0Akg, place in a reaction vessel, heat, keep Stir the reaction at a temperature of 140°C, monitor by HPLC, until the reaction of E-1,3-dimethoxy-2-isopropyl-5-(2-styryl)-benzene is complete, and the reaction time is 20h to stop the reaction;
[0054] (2) Take 13.0AL tetrahydrofuran, 9.0AL drinking water and 0.28L sulfuric acid, put them in the extraction container, stir, transfer the reaction solution in step (2) to the extraction container while it is hot, stir, let it stand, and discard the lower layer water phase; add 8.5A L of drinking water and 0.14A L of sulfuric acid, stir, let stand, discard the lower water phase; add 4.0A L of drinking water, ...
Embodiment 3
[0059] As an embodiment of the preparation method of the phenylene Moder impurity described in the present invention, the preparation method of the Benzene Moder impurity described in this embodiment comprises the following steps:
[0060] (1) Take A kg of E-1,3-dimethoxy-2-isopropyl-5-(2-styryl)-benzene and 2.0 Akg of pyridine hydrochloride, place it in a reaction vessel, heat, Keep the temperature at 220°C, stir the reaction, monitor by HPLC, until the reaction of E-1,3-dimethoxy-2-isopropyl-5-(2-styryl)-benzene is complete, the reaction time is 2h, stop the reaction ;
[0061] (2) Take 13.0A L of methyl tert-butyl ether, 9.0A L of drinking water and 0.28L of acetic acid, put them in the extraction container, stir, transfer the reaction solution in step (2) to the extraction container while it is hot, stir, and let stand , discard the lower water phase; add 8.5AL drinking water and 0.14A L acetic acid, stir, let stand, discard the lower water phase; add 4.0A L drinking wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com