Preparation method and application of anticoagulant copolymer
A copolymer and anticoagulant technology, applied in drug delivery, pharmaceutical formulations, prostheses, etc., can solve the problems of coagulation, microbial contamination, lack of anticoagulation, anti-infection, anti-protein adsorption and anti-thrombotic properties, etc. Improve anticoagulant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A copolymer suitable for modifying large-diameter blood vessels, the preparation method of which is as follows:
[0040] (1) Preparation of anticoagulant copolymer
[0041] The ratio of each monomer in the present embodiment is as follows:
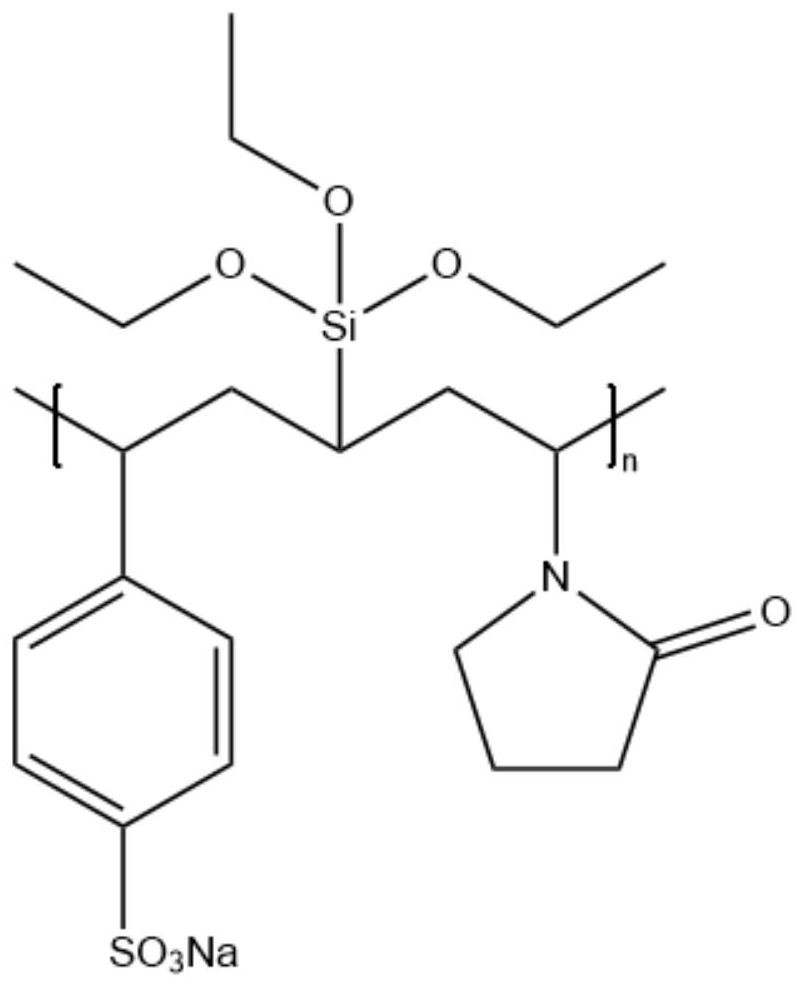
[0042] The molar ratio of sodium p-styrenesulfonate (A), N-vinylpyrrolidone (B), vinyltriethoxysilane (C) and azobisisobutyronitrile (D) is A:(B+C) :D=75:25:1.5, where B:C=75:25.
[0043]Weigh an appropriate amount of A, B, and C monomers according to the above proportions and dissolve them in 200 mL of DMAc (N,N-dimethylacetamide) solution. Under nitrogen protection, after stirring for 30 minutes, add the initiator azobisiso Butyronitrile, react at 80°C for 20 hours to make addition polymerization of three double bond-containing monomers into polymer polymers.
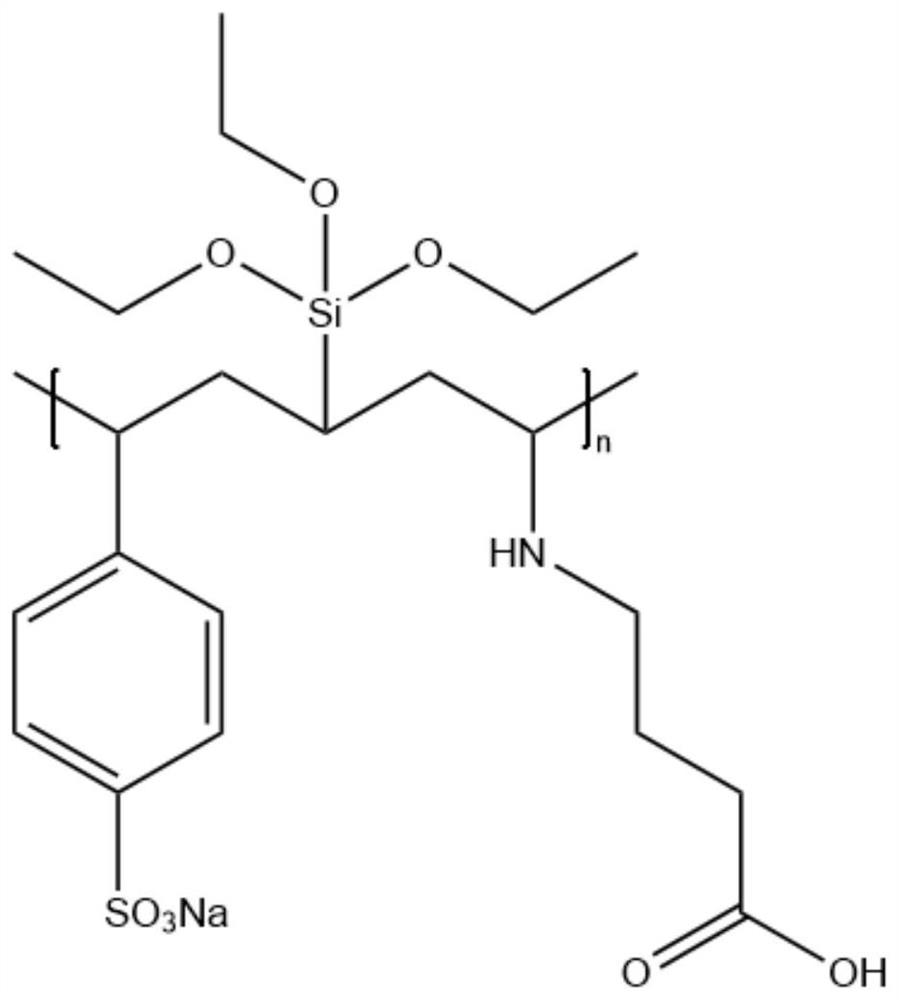
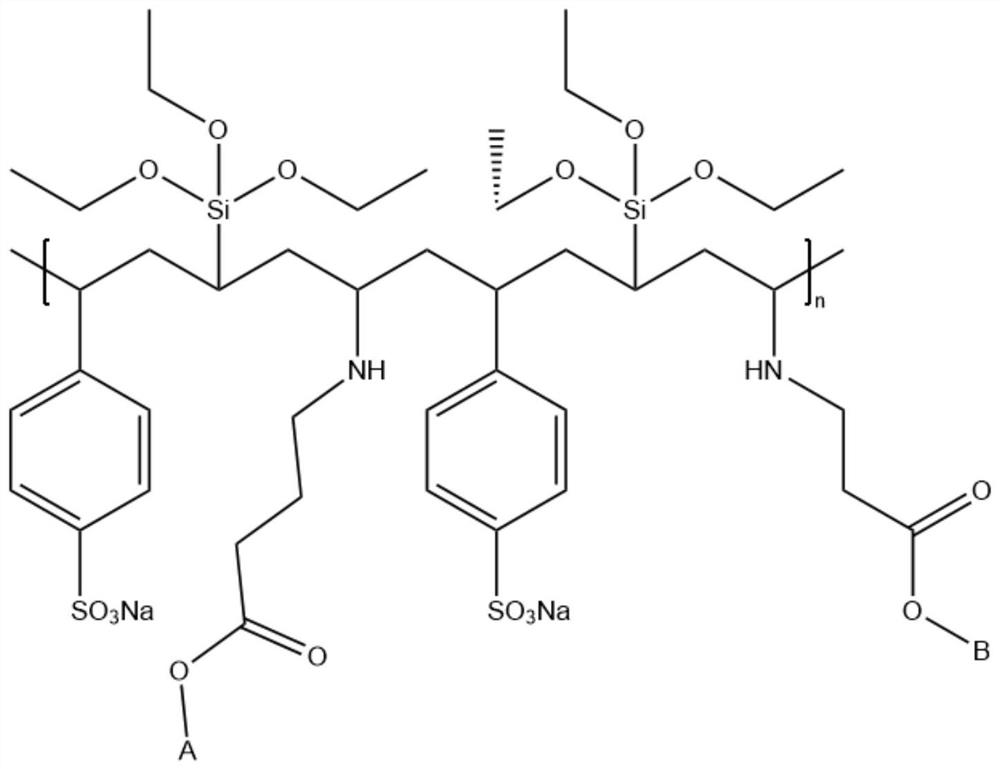
[0044] The above-mentioned polymer was heated in an aqueous solution of 5wt% sodium hydroxide and N,N-dimethylacetamide (the mass ratio of N,N-dimethylacetamide to water...
Embodiment 2
[0057] A copolymer suitable for modifying small-diameter blood vessels, the preparation method of which is as follows:
[0058] (1) Preparation of anticoagulant copolymer
[0059] The ratio of each monomer in the present embodiment is as follows:
[0060] The molar ratio of sodium p-styrenesulfonate (A), N-vinylpyrrolidone (B), vinyltriethoxysilane (C) and azobisisobutyronitrile (D) is A:(B+C) :D=25:75:1.5, where B:C=75:25.
[0061] Weigh an appropriate amount of A, B, and C according to the above ratio and dissolve them in 200 mL of DMAc solution. Under nitrogen protection, after stirring for 30 minutes, add the initiator azobisisobutyronitrile, and react at 80°C for 20 hours to make 3 Polymerization of monomers containing double bonds into polymers.
[0062] The polymerized polymer was heated in an aqueous solution of 5% sodium hydroxide and N,N-dimethylacetamide (the mass ratio of N,N-dimethylacetamide to water was 1:1) for 1 h, The pyrrolidone five-membered ring of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com