Method for preparing degradable polymer based on light control of spiropyrane, and application
A technology for degrading polymers and spiropyran, which is applied in the field of light-controlled preparation of degradable polymers based on spiropyran, and can solve problems such as regulation that has not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
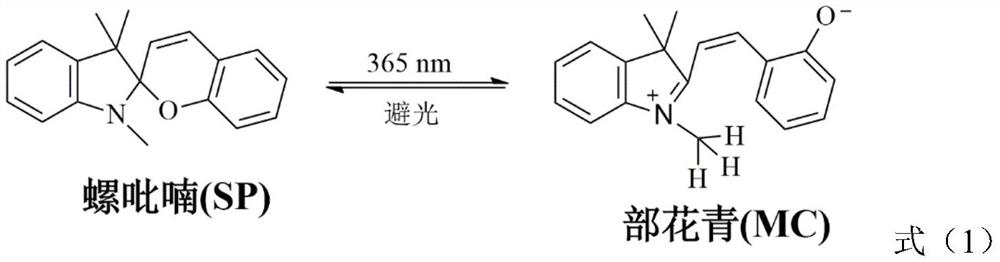
[0030] 1. 1,3,3-trimethyl-2-methyleneindoline (1.836g, 10.59mmol) was added dropwise in the ethanol solution containing salicylaldehyde (1.239g, 10.14mmol), and then Reflux for 24h under a high-purity nitrogen atmosphere. Evaporate in vacuo to obtain pink crystals, dissolve dichloromethane and wash with deionized water 3 times, dry the separated dichloromethane with anhydrous magnesium chloride for 2 h, filter, remove solvent, and dry in vacuo to obtain pink solid spiropyril mutter;
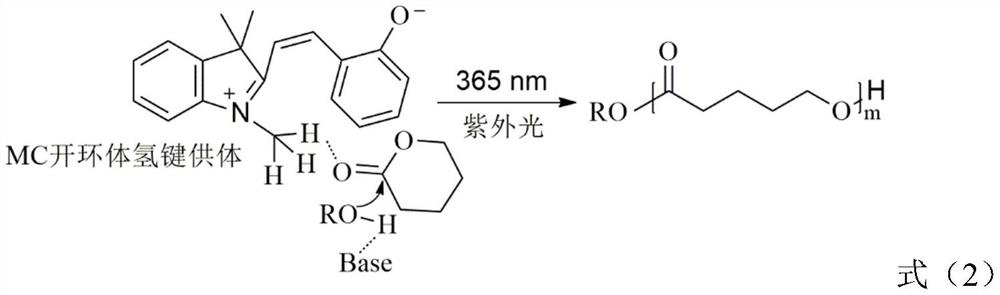
[0031] 2. Under the protection of high-purity argon, add spiropyran (35.3mg, 0.15mmol), initiator benzyl alcohol (10.8μL, 0.1mmol), monomer Lactide ( LA) (720mg, 5mmol), solvent dichloromethane (1.5mL), organic weak base hydrogen bond acceptor N,N-dimethylaminocyclohexane (32mg, 0.1mmol). React at room temperature for 1-2 hours, irradiate the reaction with ultraviolet light (365nm, 50w), take a sample after the polymerization reaction is completed, and use nuclear magnetic analysis to obtain a ...
Embodiment 2
[0034] The difference from Example 1 is that: the light time is 6 hours, and samples are taken after the reaction, and the conversion rate of LA monomer is calculated by NMR to be 51.8%. The resulting polymerization product is polylactide
Embodiment 3
[0036] The difference from Example 1 is that: the light time is 12 hours, and samples are taken after the reaction is completed, and the conversion rate of LA monomer is calculated by NMR to be 81.8%. The resulting polymerization product is polylactide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com