Photosensitive covalent organic framework material as well as preparation method and application thereof
A covalent organic framework and photosensitivity technology, applied in the field of preparation of covalent organic framework materials, can solve the problems of cumbersome operation, harsh synthesis conditions and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
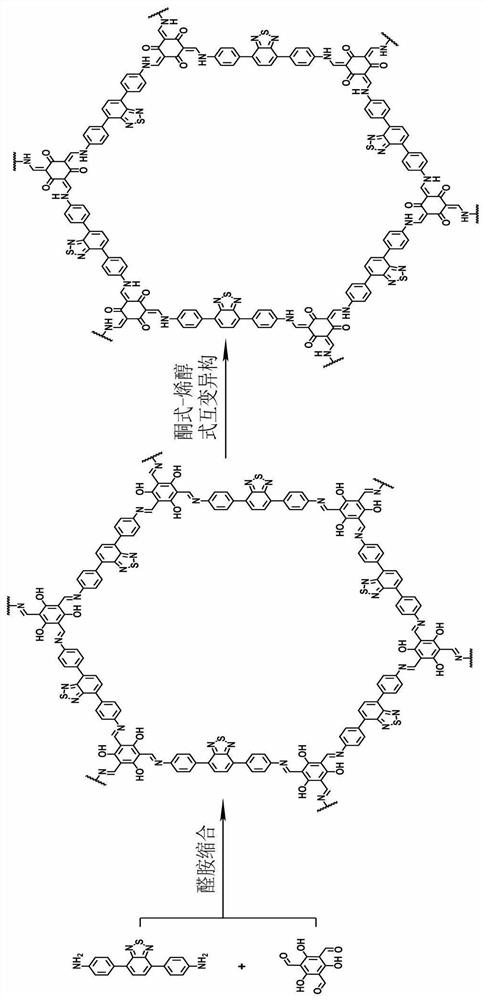
[0038] The invention provides a method for preparing a photosensitive covalent organic framework material, comprising the following steps:
[0039] Mix 4,7-bis(4-aminophenyl)-2,1,3-benzothiadiazole, trialdehyde phloroglucinol and a solvent for condensation reaction to obtain the photosensitive covalent organic framework Material.
[0040] In the present invention, unless otherwise specified, the raw materials used are all preferably commercially available products.
[0041]In the present invention, the molar ratio of 4,7-bis(4-aminophenyl)-2,1,3-benzothiadiazole to trialdehyde phloroglucinol is preferably (1-10): 1, more preferably 3:2.
[0042] In the present invention, the solvent is preferably a mixture of o-dichlorobenzene, n-butanol and aqueous acetic acid; the concentration of the aqueous acetic acid is preferably 6mol / L; The volume ratio is preferably 1:(0.25-4):(0.1-0.3), more preferably 1:1:0.2.
[0043] In the present invention, the amount ratio of the solvent to...
Embodiment 1
[0058] A method for preparing a photosensitive COFs material, comprising the steps of:
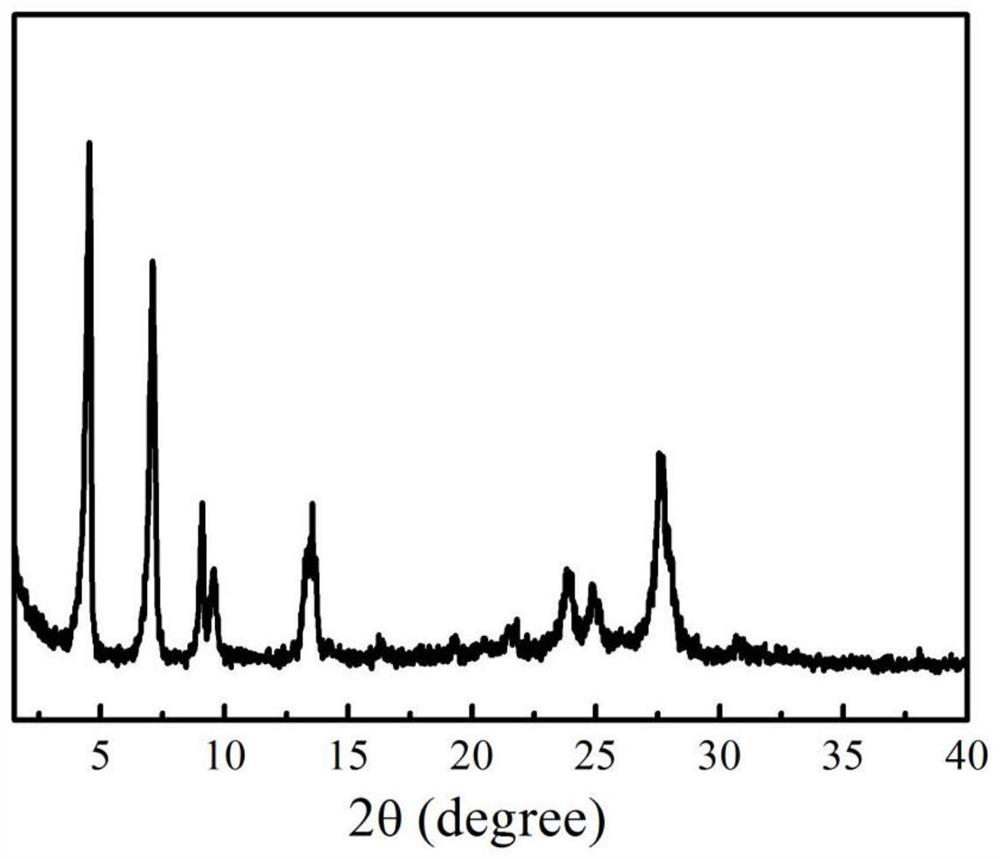
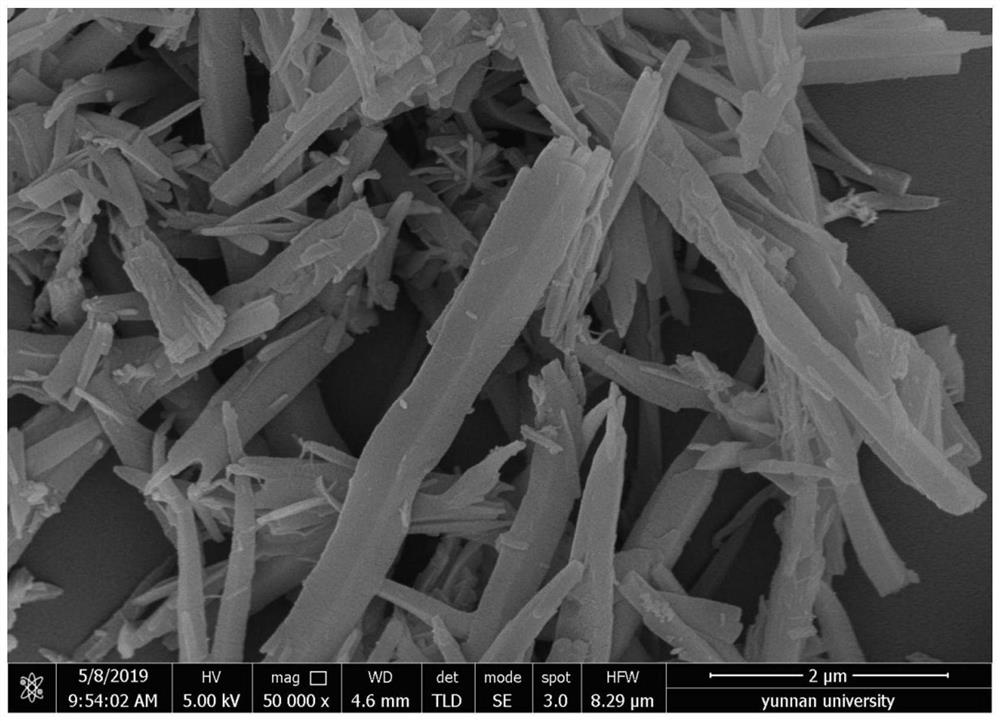
[0059] Add organic monomer 4,7-bis(4-aminophenyl)-2,1,3-benzothiadiazole (238 mg, 0.75 mmol) and trialdehyde phloroglucinol ( 105mg, 0.5mmol), add 10mL o-dichlorobenzene, 10mL n-butanol, 2mL 6mol / L acetic acid aqueous solution, disperse evenly in 100W ultrasonic for 5min, then carry out condensation reaction at 25℃, 0.101MPa, 300rpm for 3 days .
[0060] The obtained condensation reaction feed liquid was centrifuged (12000 rpm), and the obtained precipitate was washed 5 times with tetrahydrofuran and 3 times with acetone, and vacuum-dried at 120° C. for 1 day to obtain a photosensitive COFs material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com