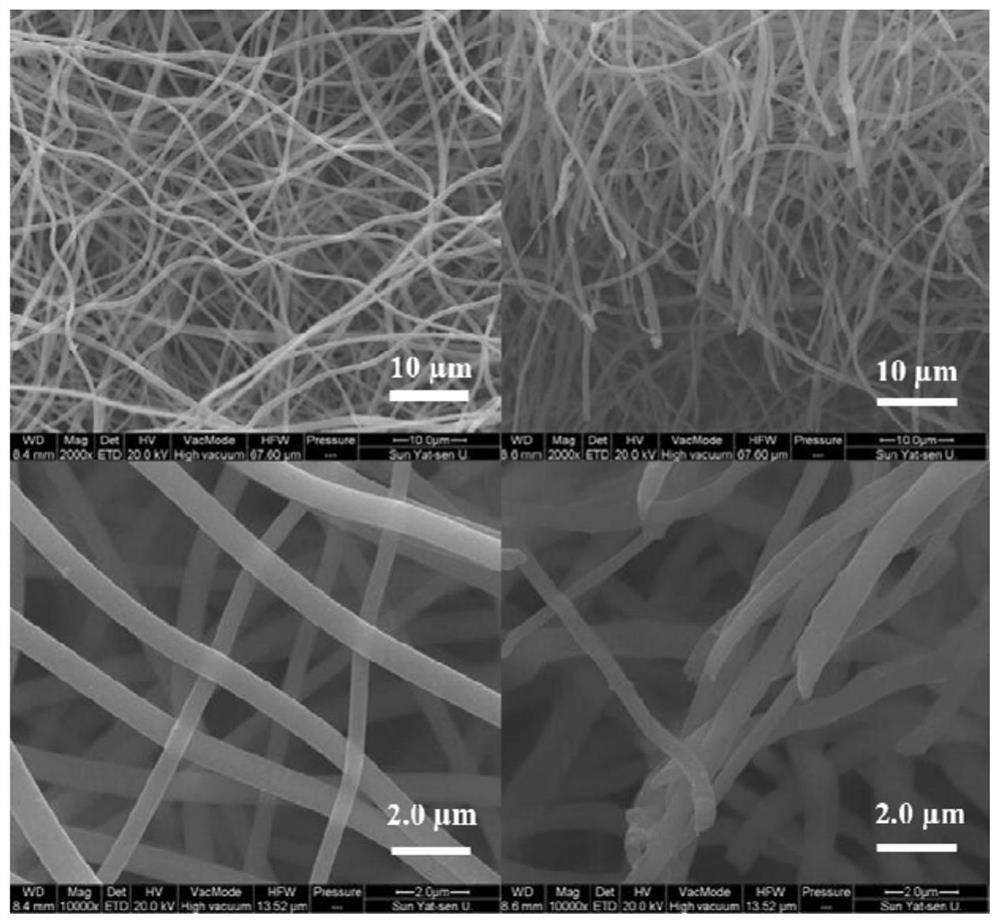
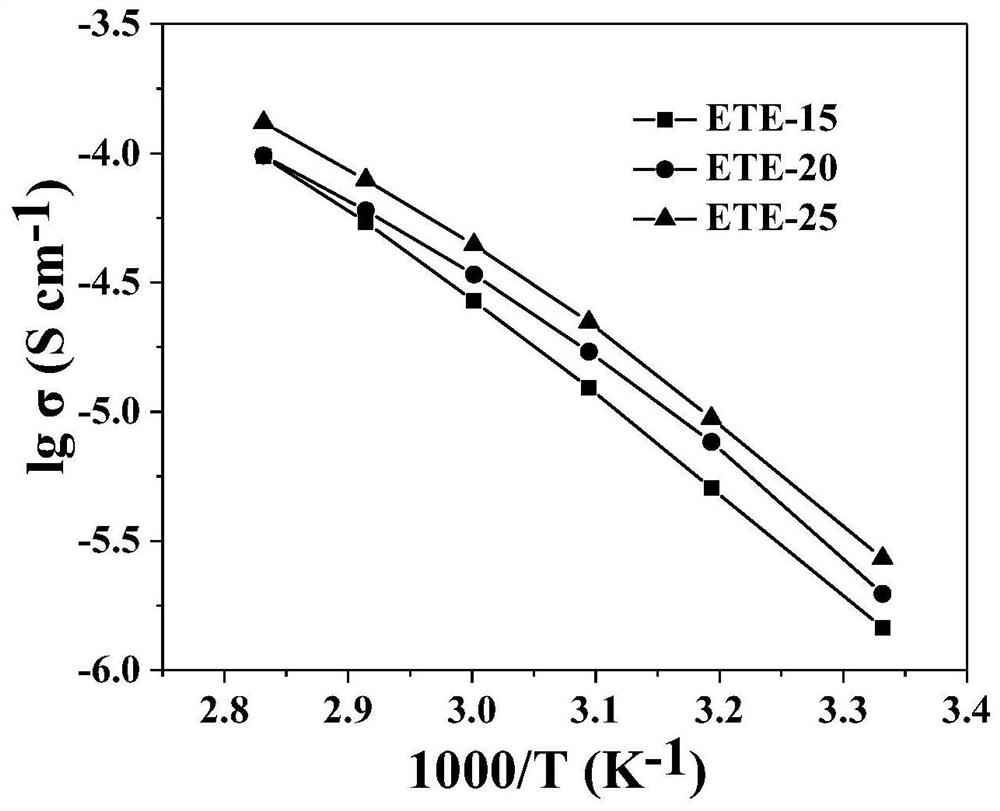
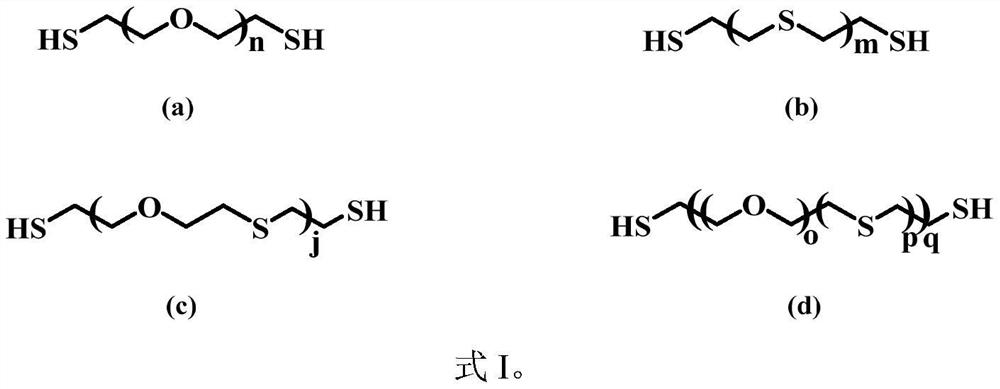
Ultrathin polymer electrolyte membrane based on porous polyimide and preparation method thereof
A technology of polyimide membrane and electrolyte membrane, which is applied in the direction of solid electrolyte, non-aqueous electrolyte, non-aqueous electrolyte battery, etc., can solve the problems of reducing mechanical strength, increasing membrane rupture, safety accidents, etc., and achieves low interface impedance, excellent performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of ultra-thin polymer electrolyte membrane (SPE-1)
[0036] 1,2-Ethanedithiol (EDT) and diethylene glycol divinyl ether (DGDE) were the reactive monomers, and 2,2-dimethoxy-2-phenylacetophenone (DMPA) was the photoinitiated Lithium bistrifluorosulfonyl imide (LiTFSI, formula IV (a)) is a small molecule lithium salt, pentaerythritol tetraacrylate (PET4A, formula II (a)) is a crosslinking agent, and anhydrous tetrahydrofuran (THF) is solvent.
[0037] The following operations were all carried out in a glove box under an argon atmosphere. The reaction is divided into two steps. The first step: add EDT and DGDE to a 20mL small glass bottle, then add DMPA, stir it in the dark to dissolve it completely, and initiate the reaction under 365nm ultraviolet light to prepare a sulfhydryl-terminated linear Ion-conducting polymer (formula I(d)); the second step: add LiTFSI, PET4A, THF and DMPA to the above product, stir in the dark to dissolve complete...
Embodiment 2
[0044] Embodiment 2: the preparation of ultra-thin polymer electrolyte membrane (SPE-2)
[0045]1,2-Ethanedithiol (EDT) and diethylene glycol divinyl ether (DGDE) were the reactive monomers, and 2,2-dimethoxy-2-phenylacetophenone (DMPA) was the photoinitiated Lithium perchlorate (LiClO4, formula IV (i)) is a small molecule lithium salt, hexaeugenol cyclotriphosphazene (HECTP, formula III (a)) is a crosslinking agent, and anhydrous tetrahydrofuran (THF) is a solvent .
[0046] Except that the small molecule lithium salt and the cross-linking agent were changed, the dosage and operation steps of each reagent were the same as in Example 1.
[0047] In the linear ion-conducting polymer, q is preferably 10-25.
[0048] The molar ratio of the linear ion-conducting polymer to HECTP is 3:1.
[0049] The mass proportion of the lithium salt in the ultra-thin polymer electrolyte membrane is 25%.
[0050] The added amount of DMPA is 3%.
[0051] The added amount of the solvent THF is...
Embodiment 3
[0053] Embodiment 3: the preparation of ultra-thin polymer electrolyte membrane (SPE-3)
[0054] 1,2-Ethanedithiol (EDT) and diethylene glycol divinyl ether (DGDE) were the reactive monomers, and 2,2-dimethoxy-2-phenylacetophenone (DMPA) was the photoinitiated Agent, lithium bistrifluorosulfonyl imide (LiTFSI, formula IV (a)) is a small molecule lithium salt, hexaeugenol cyclotriphosphazene (HECTP, formula III (a)) is a crosslinking agent, anhydrous tetrahydrofuran ( THF) as solvent.
[0055] Except that the cross-linking agent was changed, the dosage and operation steps of each reagent were the same as in Example 1.
[0056] In the linear ion-conducting polymer, q is preferably 10-25.
[0057] The molar ratio of the linear ion-conducting polymer to HECTP is 3:1.
[0058] The mass proportion of the lithium salt in the ultra-thin polymer electrolyte membrane is 30%.
[0059] The added amount of DMPA is 5%.
[0060] The added amount of the solvent THF is 30% of the total ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com