Enrofloxacin injection and preparation method thereof
A technology for enrofloxacin and injection, applied in the field of enrofloxacin injection and its preparation, can solve problems such as poor curative effect and easy crystallization, and achieve the effects of avoiding both symptoms and root causes, good sterilization effect, and overcoming drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
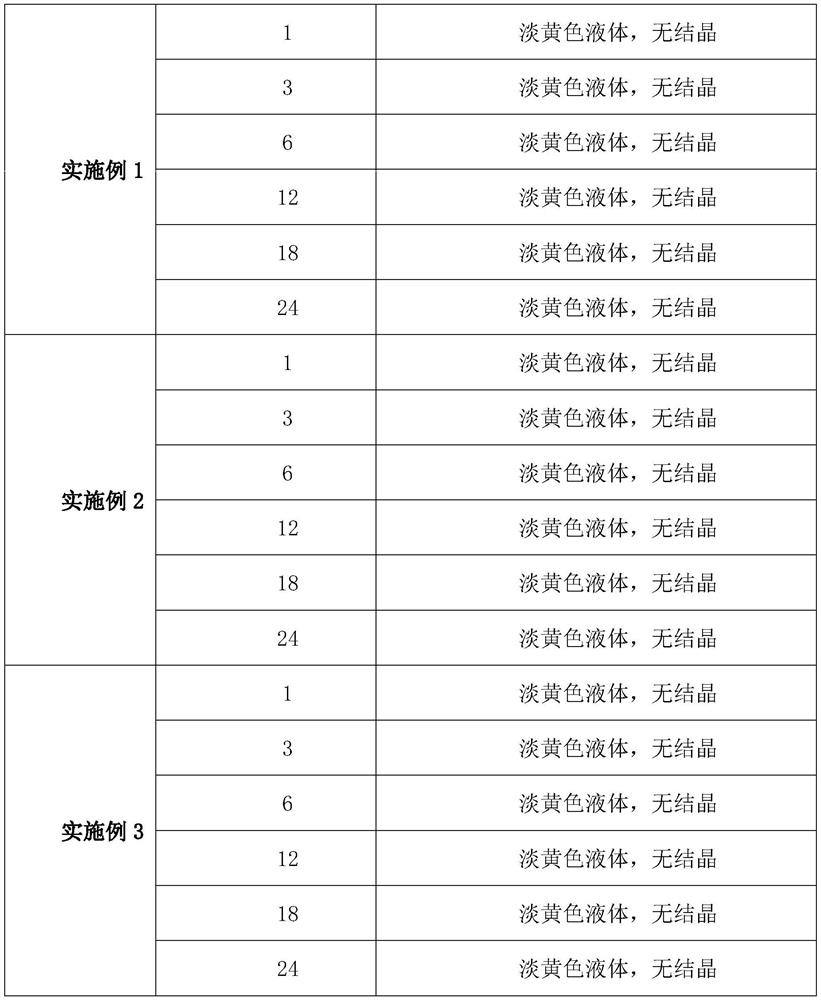
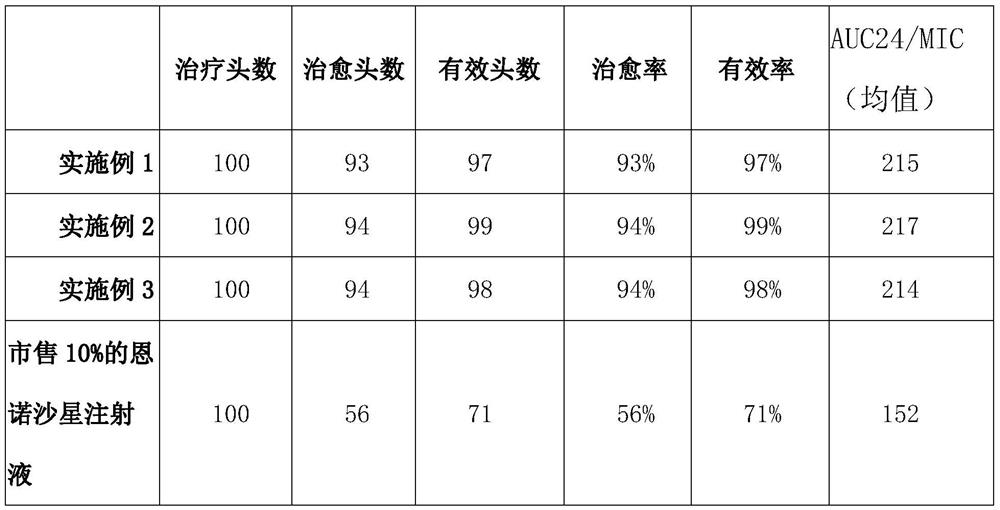
Embodiment 1
[0057] (1) Take 10 parts of enrofloxacin, 1 part of dimethylformamide and 5 parts of tributyrin, stir and heat to 60 degrees to dissolve, and obtain solution A;
[0058] (2) After mixing and dissolving 0.8 parts of nabumetone and 0.4 parts of sodium lauryl sulfate, and then stirring with 5 parts of tributyrin and heating to 60 degrees for dissolution, solution B was obtained;
[0059] (3) Stir and heat 3 parts of glycerin and 10 parts of soybean oil for injection to 50 degrees to obtain solution C;
[0060] (4) Mix solution A, solution B and solution C to obtain solution D;
[0061] (5) Adjust the pH of solution D to 9.5 with ethanolamine, add water for injection to the full amount to obtain enrofloxacin injection, and finally filter the obtained enrofloxacin injection with a 0.45 μm microporous membrane, and pour Seal in an ampoule and sterilize at 110°C for 40 minutes under circulating steam.
Embodiment 2
[0063] (1) Take 13 parts of enrofloxacin, 2 parts of dimethylformamide and 6 parts of tributyrin, stir and heat to 60 degrees to dissolve, and obtain solution A;
[0064] (2) Mix and dissolve 1 part of nabumetone and 0.5 part of sodium lauryl sulfate, then stir and heat to 60 degrees with 7 parts of tributyrin to dissolve, and obtain solution B;
[0065] (3) 5 parts of glycerin and 15 parts of soybean oil for injection were stirred and heated to 50 degrees to obtain solution C;
[0066] (4) Mix solution A, solution B and solution C to obtain solution D;
[0067] (5) Use ethanolamine to adjust the pH of solution D to 10, add water for injection to the full amount to obtain enrofloxacin injection, and finally filter the obtained enrofloxacin injection with a 0.45 μm microporous membrane, and pour Seal in an ampoule and sterilize at 110°C for 40 minutes under circulating steam.
Embodiment 3
[0069] (1) Take 15 parts of enrofloxacin, 3 parts of dimethylformamide and 7 parts of tributyrin, stir and heat to 60 degrees to dissolve, and obtain solution A;
[0070] (2) After mixing and dissolving 1.2 parts of nabumetone and 0.6 parts of sodium lauryl sulfate, and then stirring with 8 parts of tributyrin and heating to 60 degrees for dissolution, solution B was obtained;
[0071] (3) 6 parts of glycerin and 20 parts of soybean oil for injection were stirred and heated to 50 degrees to obtain solution C;
[0072] (4) Mix solution A, solution B and solution C to obtain solution D;
[0073] (5) Use ethanolamine to adjust the pH of solution D to 10.5, add water for injection to the full amount to obtain enrofloxacin injection, and finally filter the obtained enrofloxacin injection with a 0.45 μm microporous membrane, and pour Seal in an ampoule and sterilize at 110°C for 40 minutes under circulating steam.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com