A carbon-11 ( 11 c) Radiopharmaceuticals and their preparation methods and applications
A radiopharmaceutical and radioactive technology, applied in the field of medicine, achieves the effects of simple synthesis, fast and efficient labeling, and good targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] targeting fibroblast activation protein (FAP) 11 C-labeled positron radiopharmaceuticals 11 The preparation method of C-FAPI-01 comprises the following steps:
[0053] Step 1. Synthesis reaction: use the accelerator of Sumitomo 10MV in Japan to pass the nuclear reaction 14 N(p,α) 11 C production 11 CO 2 , target beam current is 50μA, bombardment 30min generates 30GBq 11 CO 2 ; 11 CO 2 Use high-purity nitrogen as a carrier gas to transfer it to a temperature of -10°C, 300 μL, a concentration of 0.3M lithium aluminum hydride in tetrahydrofuran solution for 3 minutes, and then heat to 150°C to evaporate tetrahydrofuran to dryness (about 1.5 minutes), and then Add 0.3 milliliters of concentration and be 57% hydroiodic acid, heat to 180 ℃ and react for 3 minutes, the 11 CH 3 I gas is obtained directly by passing through a glass tube loaded with silver trifluoromethanesulfonate at a temperature of 200°C 11 C methyl trifluoromethanesulfonate ( 11 CH 3 OTf), direct...
Embodiment 2
[0057] targeting fibroblast activation protein (FAP) 11 C-labeled positron radiopharmaceuticals 11 The preparation method of C-FAPI-02 comprises the following steps:
[0058] Step 1. Synthesis reaction: use the accelerator of Sumitomo 10MV in Japan to pass the nuclear reaction 14 N(p,α) 11 C production 11 CO 2 , target beam current is 50μA, bombardment 20min generates 25GBq 11 CO 2 ; 11 CO 2 Use high-purity nitrogen as a carrier gas to transfer it to a temperature of -12°C, 500 μL, a concentration of 1M lithium aluminum hydride tetrahydrofuran solution for 2 minutes, then heat to 150°C to evaporate tetrahydrofuran to dryness (about 2 minutes), and then add 0.5 milliliters of concentration is 57% hydroiodic acid, is heated to 180 ℃ and reacts for 4 minutes, generates during the reaction 11 CH 3 I gas is obtained directly by passing through a glass tube loaded with silver trifluoromethanesulfonate at a temperature of 200°C 11 C Methyl trifluoromethanesulfonate ( 11 C...
Embodiment 3
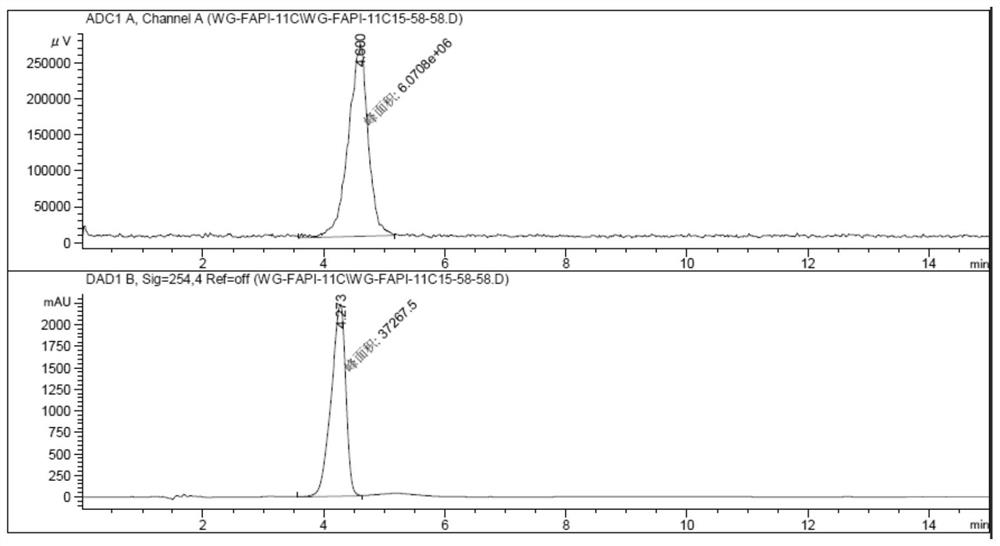
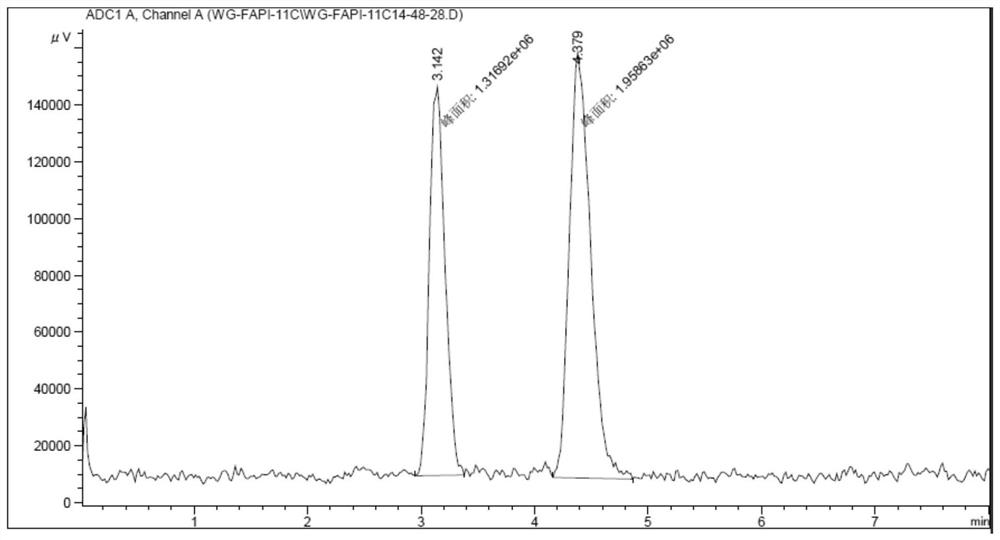
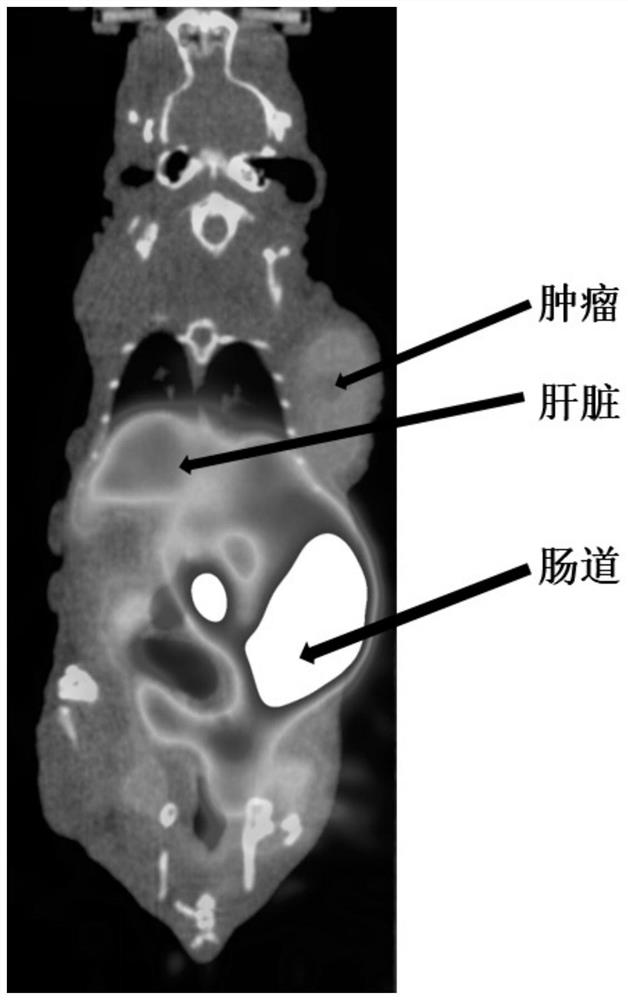
[0061] The target fibroblast activation protein (FAP) obtained in embodiment 1 11 C-labeled positron radiopharmaceuticals 11 C-FAPI-01 was used as a PET imaging agent in the imaging of tumor model mice. will be 7.4MBq 11 C-FAPI-01 was injected into mice through the tail vein, and small animal PET imaging was performed after 30 minutes of drug metabolism, as shown in image 3 Shown, visible radiopharmaceuticals 11 C-FAPI-01 is highly uptaked in tumor sites in high-expression FAP model mice.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com