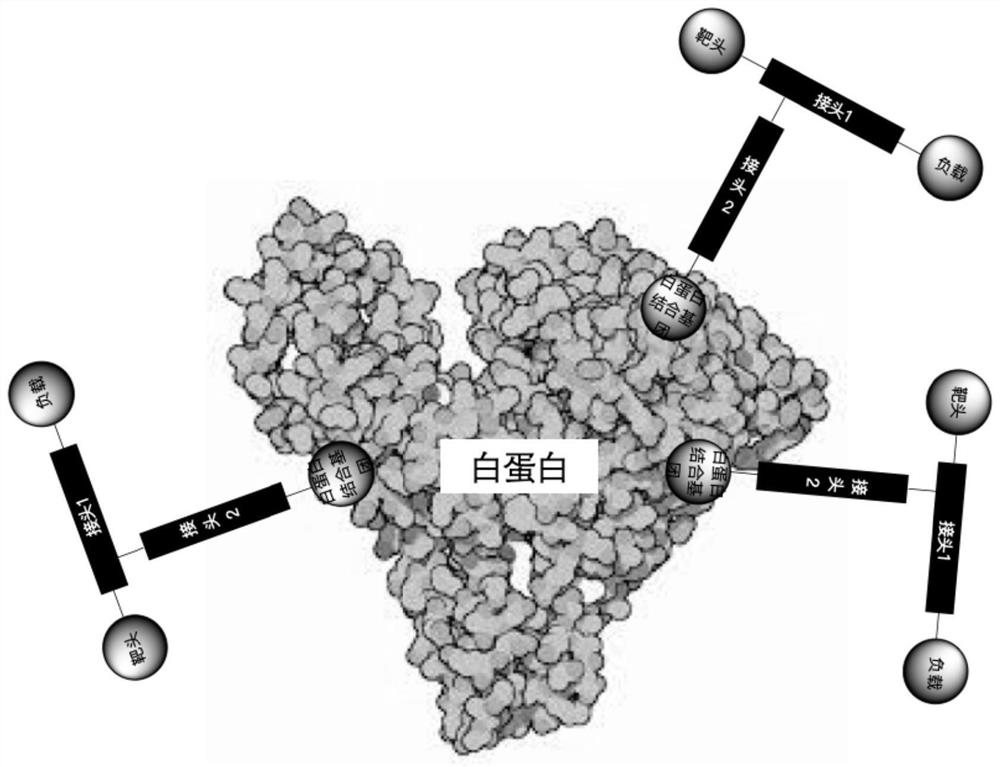
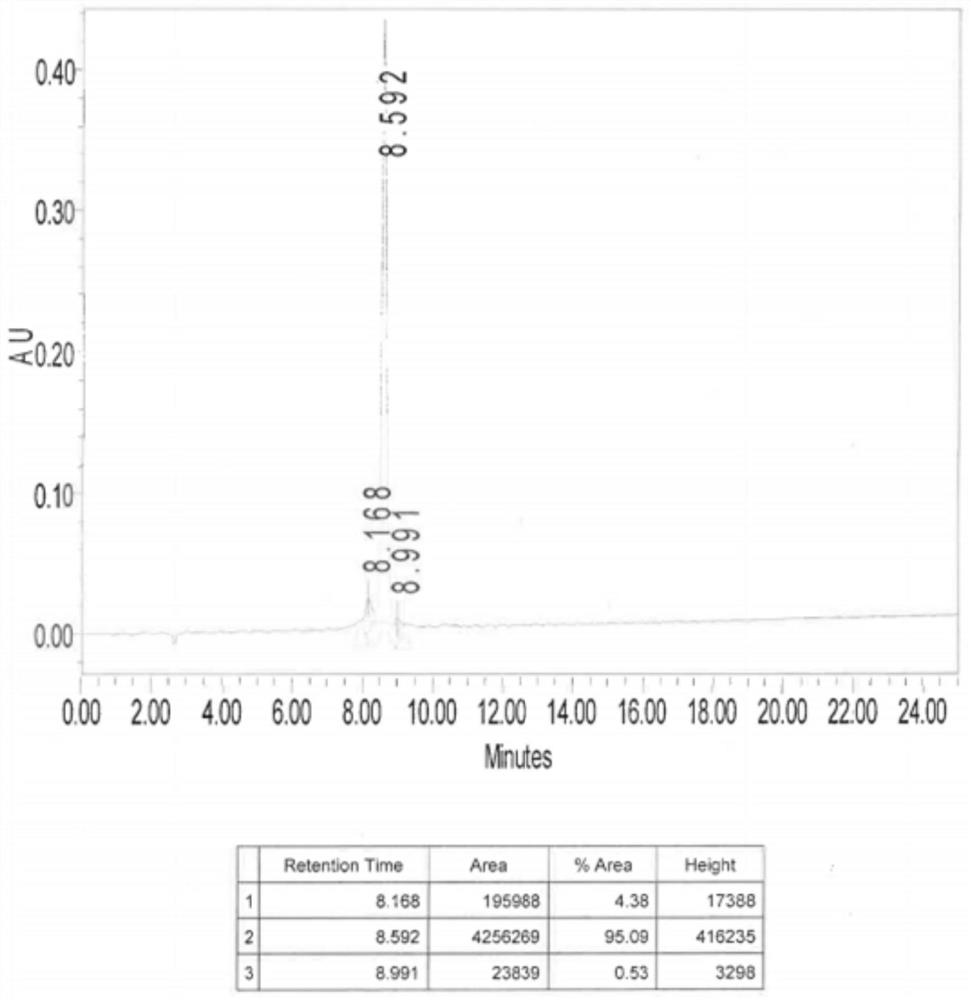
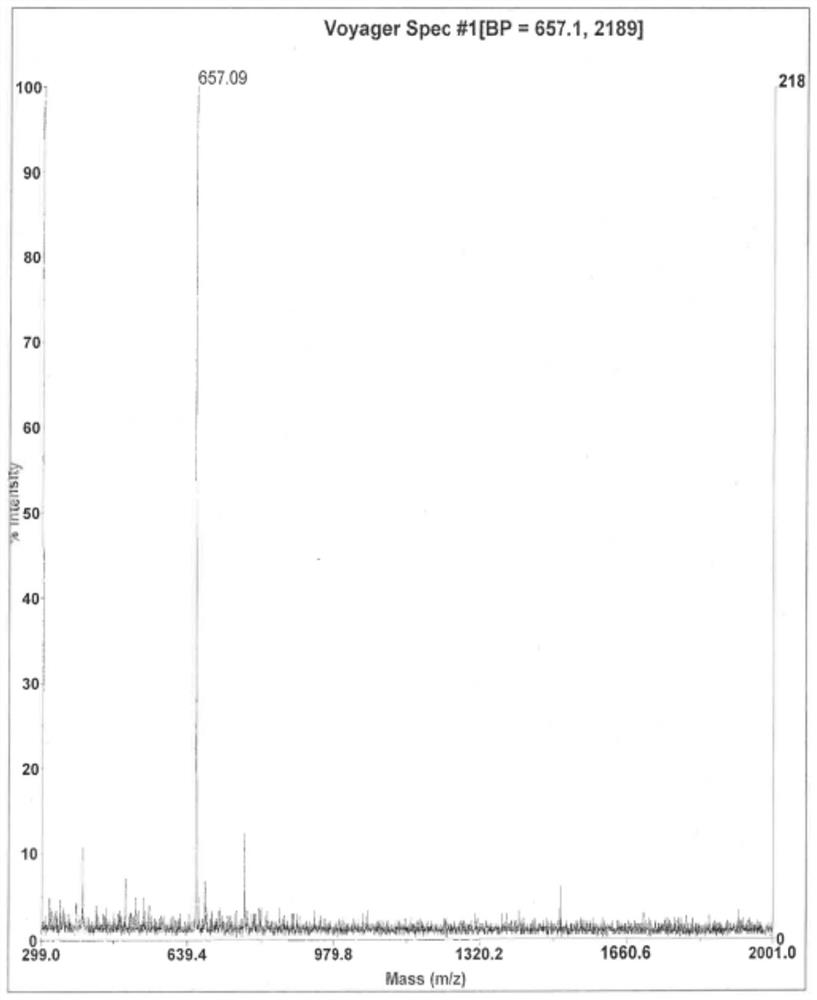
PSMA-targeted fluorescent molecular probe as well as preparation method and application thereof
A technology of fluorescent molecular probes and small molecule inhibitors, applied in the field of fluorescent molecular probes targeting PSMA and its preparation, can solve the problems of poor tumor tissue permeability, short residence time, slow clearance, etc., and achieve tumor enrichment time Long, enhanced binding ability, excellent TBR effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] The synthetic method of embodiment 1 fluorescent molecular probe (compound 6)
[0114]
[0115] Compound 2:
[0116] Resin activation: Compound 1 (Fmoc-Lys(Dde)-Wang resin, purchased from Nanjing Peptide Biotechnology Co., Ltd., R61124, 0.3-0.8mmol / g, 100-200mesh, 1g) was first mixed with hexahydropyridine / DMF Solvent I (volume ratio 25%:75%) removes the N-terminal Fmoc protecting group to make the N-terminal a free amino group.
[0117] Adding amino acid: Dissolve N,N disuccinimidyl carbonate (3.2mmol), DIPEA (10mmol) and H-Glu(OtBu)-OtBu (3.2mmol) in 20ml DMF, add to the above 12-hour Resin (de-Fmoc protected compound 1), reacted for 24 hours to obtain compound 2.
[0118] Compounds 3, 4, and 5 adopt the same grafting operation as above:
[0119] Compound 3: After removing the side chain Dde protecting group of Lys on the resin (ie compound 2) with 2% hydrazine hydrate / DMF solution (hydrazine hydrate: DMF = 2%: 98%), add 3 times the equivalent of Fmoc-2-Nal- OH...
Embodiment 2
[0125] The synthetic method of embodiment 2 fluorescent molecular probes (compound 11 and 12)
[0126]
[0127] Compound 7: Use 25% hexahydropyridine / DMF (volume ratio, the same meaning as above) to remove the Fmoc protecting group in compound 3 to make the N-terminus of 2-Nal a free amino group, and add 3 times the equivalent of N-Fmoc-trans-4-aminomethyl Base cyclohexane carboxylic acid / HOBt / DIC (that is to say, the consumption of N-Fmoc-trans-4-aminomethyl cyclohexane carboxylic acid, HOBt, DIC is 3 times of the resin molar weight) and reacted for 24 hours at normal temperature, A grafting reaction was performed to introduce trans-4-aminomethylcyclohexanecarboxylic acid.
[0128]
[0129] Compound 8: Use 25% hexahydropyridine / DMF (volume ratio) to remove the Fmoc protecting group on compound 7, add 3 times the equivalent of N-Fmoc-N'-[1-(4,4-dimethyl-2,6- Dioxocyclohexylidene)ethyl]-lysine / HOBt / DIC (that is, N-Fmoc-N'-[1-(4,4-dimethyl-2,6-dioxocyclohexyl The amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com