Synthesis method of cytochalasin compound flavipesine A
A technology of cytochalasin and synthesis method, which is applied in the field of synthesis of cytochalasin compound flavipesineA, which can solve the problems of high cost, long cycle and high price, and achieve the effects of low cost, short production cycle and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
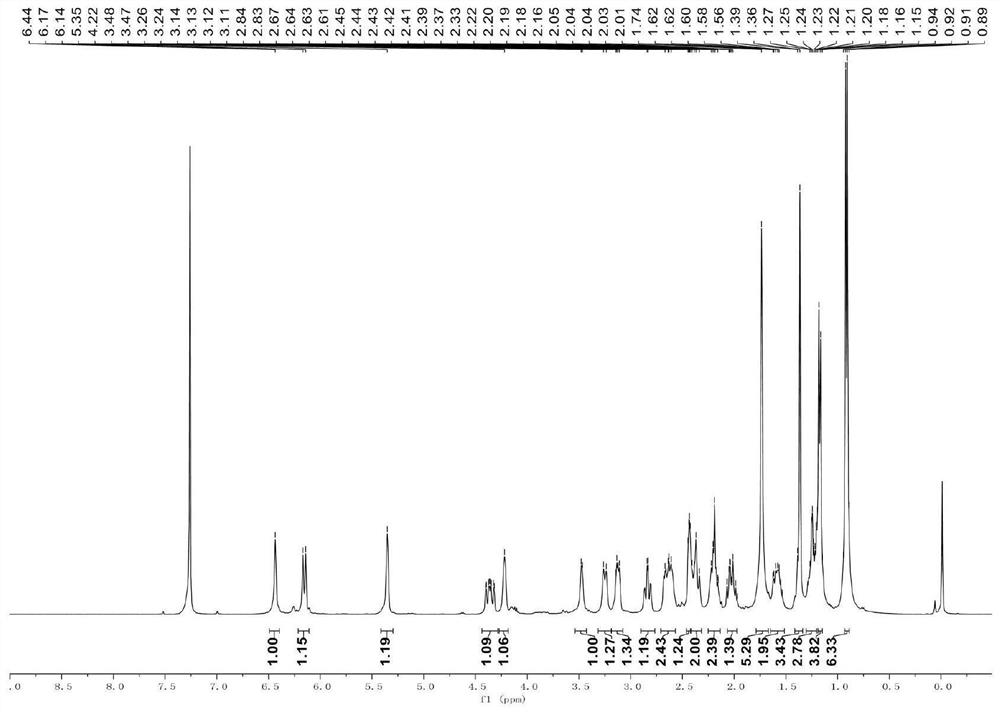
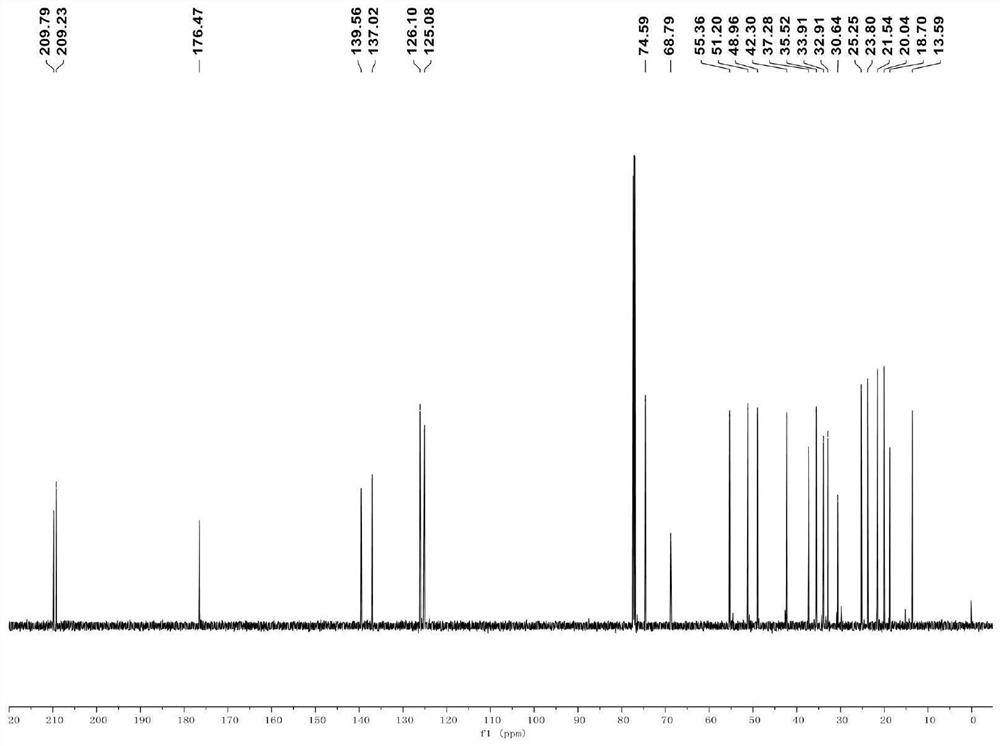
[0032] Embodiment 1 of the present invention provides the synthetic method of compound B used in the present invention, specifically as follows:
[0033]
[0034] Mix p-toluenesulfonic acid (2.27mmol) and 2,2,6,6-tetramethylpiperidine oxide (2.34mmol) in 15mL dichloromethane solution under ice bath, and continue stirring (400rpm) for 10min under ice bath Finally, the dichloromethane solution of this mixture was added to the 7 mL dichloromethane solution of compound D (0.38 mmol), and the stirring (400 rpm) was continued for 1 h under ice bath, and then the reaction was quenched with 10 mL saturated aqueous sodium bicarbonate solution under ice bath (10min), extracted with ethyl acetate at room temperature, washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered under reduced pressure (500mbar), and concentrated under reduced pressure (150mbar), the crude product obtained was purified by silica gel column Analysis and purification (V ethy...
Embodiment 2
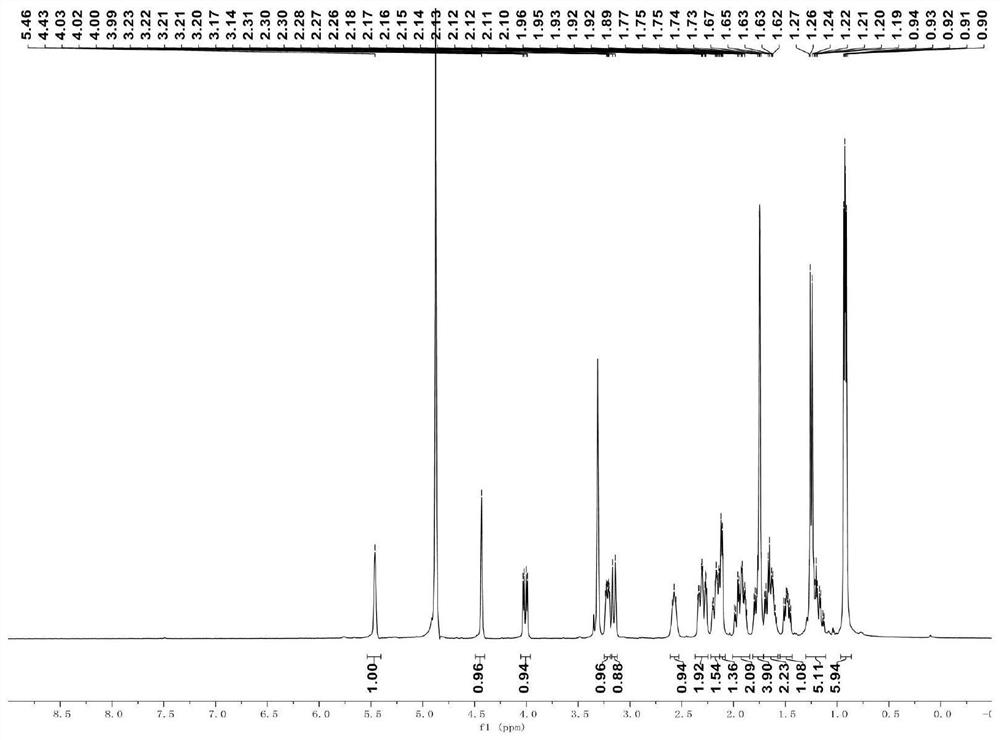
[0040] A kind of synthetic method of cytochalasin compound flavipesine A, its synthetic route is specifically as follows:
[0041]
[0042] Specific steps are as follows:
[0043] (1) Dissolve compound B (0.075mmol) in 1.5mL of anhydrous tetrahydrofuran at room temperature, add zinc powder (0.90mmol) and ammonium acetate (1.05mmol), then stir (450rpm) at 40°C for 2h, and then At room temperature, filter through diatomaceous earth (500mbar) and concentrate under reduced pressure (150mbar) to obtain the crude product, which is purified by silica gel column chromatography (eluent: V ethyl acetate: V petroleum ether = 50:50) to obtain intermediate C (26.9mg, yield rate is 89%);
[0044] (2) Dissolve Intermediate C (0.05mmol) in 1mL of anhydrous tetrahydrofuran at room temperature, add scandium trifluoromethanesulfonate (0.25mmol), stir (450rpm) for 2h, and then add 1mL of saturated sodium bicarbonate at room temperature The reaction was quenched with aqueous solution (10min),...
Embodiment 3
[0046]
[0047] (1) Dissolve compound B (0.075mmol) in 0.15mL anhydrous tetrahydrofuran at room temperature, add zinc powder (0.75mmol) and ammonium acetate (0.90mmol), then stir (450rpm) at 40°C for 2h, and then Filtration through diatomaceous earth (500mbar) at room temperature and concentration under reduced pressure (150mbar) gave the crude product, which was purified by silica gel column chromatography (V ethyl acetate: V petroleum ether = 50:50) to obtain intermediate C (22mg, yield 73%);
[0048] (2) Dissolve Intermediate C (0.05mmol) in 0.1mL of anhydrous tetrahydrofuran at room temperature, add scandium trifluoromethanesulfonate (0.20mmol), stir (450rpm) for 2h, and then add 1mL of saturated bicarbonate The reaction was quenched with sodium aqueous solution (10min), extracted with ethyl acetate, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered under reduced pressure (500mbar), and concentrated under reduced press...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap