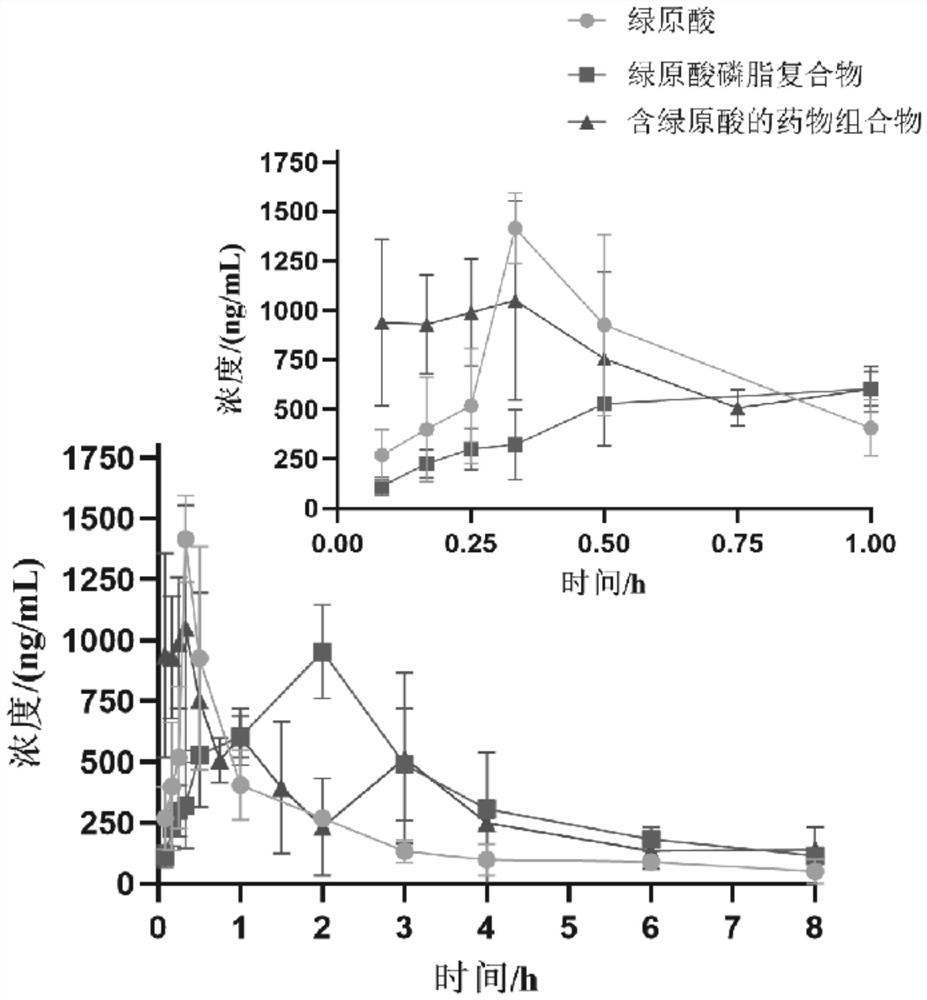
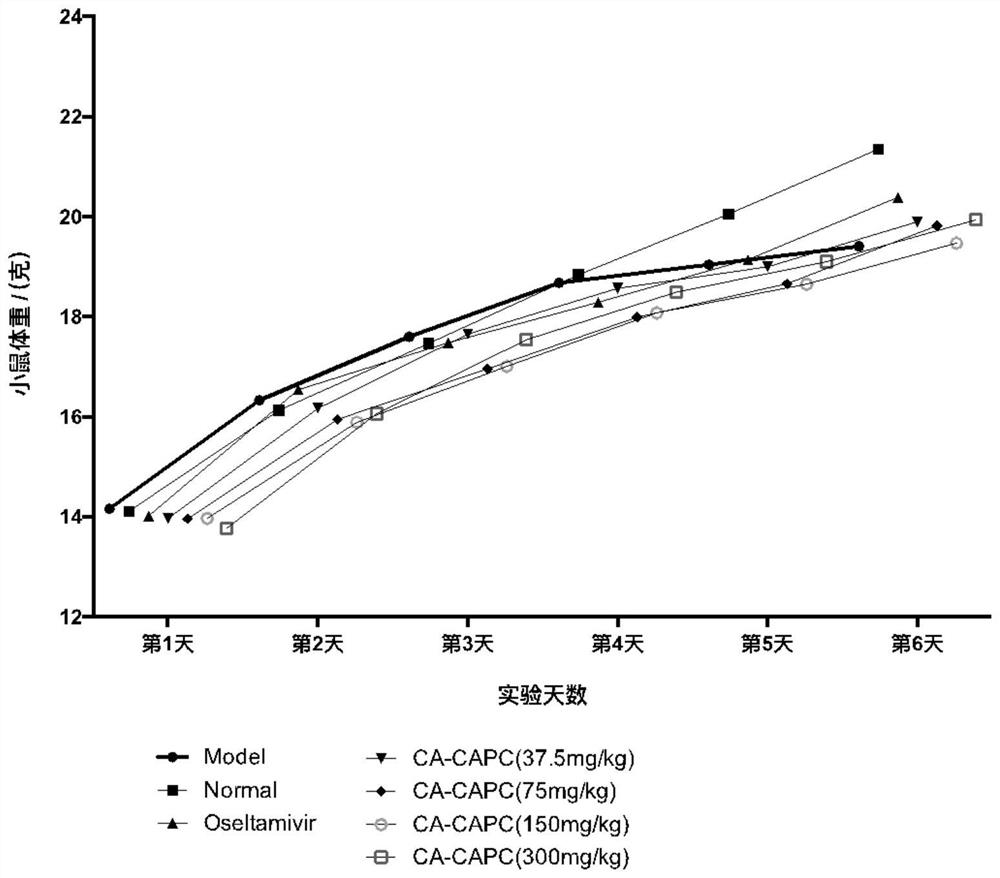
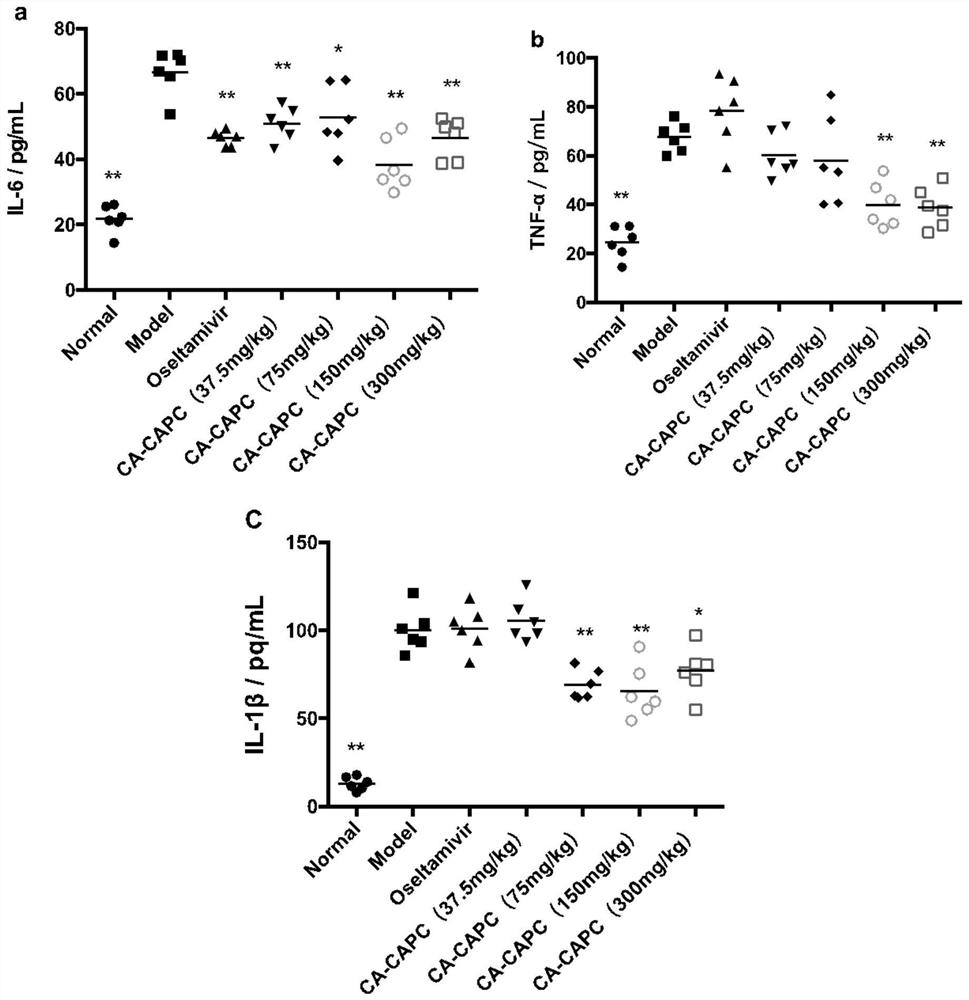
Application of pharmaceutical composition containing chlorogenic acid in preparation of antiviral drugs
An antiviral drug, chlorogenic acid technology, applied in the field of medicine, can solve the problems of toxic and side effects of organs such as liver and kidney, difficult to cure chronic diseases, and low clinical efficacy, achieve high oral bioavailability, and improve oral pharmacokinetic parameters. , the effect of reducing lung index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preparation method of a pharmaceutical composition containing chlorogenic acid, comprising the steps of:
[0041] (1) Weigh soybean lecithin and the first part of chlorogenic acid, put them in a container, add ethanol aqueous solution with a volume concentration of 75%, stir at 30°C for 2h, and recover ethanol under reduced pressure (50°C, -0.08Mpa). Drying under reduced pressure, crushing the dried solids, and passing through a No. 5 sieve to obtain the chlorogenic acid phospholipid complex;
[0042] (2) Weighing the second portion of chlorogenic acid and the chlorogenic acid phospholipid complex obtained in step (1), mixing them, and stirring evenly to obtain a pharmaceutical composition containing chlorogenic acid.
[0043] The mass ratio of soybean lecithin, the first chlorogenic acid and 75% ethanol aqueous solution is 5:5:1000.
[0044] The mass ratio of the second chlorogenic acid to the chlorogenic acid-phospholipid complex obtained in step (1) is 1:2.
[00...
Embodiment 2
[0047] A preparation method of a pharmaceutical composition containing chlorogenic acid, comprising the steps of:
[0048] (1) Weigh dimyristoylphosphatidylethanolamine and the first part of chlorogenic acid, put them in a container, add ethanol aqueous solution with a volume concentration of 90%, stir at 35°C for 1h, and reduce pressure (50°C, -0.08Mpa) Recover ethanol, dry the solid under reduced pressure, pulverize the dried solid, and pass through a No. 6 sieve to obtain the chlorogenic acid phospholipid complex;
[0049](2) Weighing the second portion of chlorogenic acid and the chlorogenic acid-phospholipid complex obtained in step (1), mixing them, and stirring evenly to obtain a pharmaceutical composition containing chlorogenic acid.
[0050] The mass ratio of dimyristoylphosphatidylethanolamine, the first part of chlorogenic acid and the aqueous ethanol solution with a volume concentration of 90% is 10:2:4000.
[0051] The mass ratio of the second chlorogenic acid to...
Embodiment 3
[0053] A preparation method of a pharmaceutical composition containing chlorogenic acid, comprising the steps of:
[0054] (1) Weigh dimyristoylphosphatidylglycerol and the first part of chlorogenic acid, put them in a container, add an aqueous ethanol solution with a volume concentration of 80%, stir at 20°C for 2h, and reduce pressure (50°C, -0.08Mpa) Recover ethanol, dry the solid under reduced pressure, pulverize the dried solid, and pass through a No. 4 sieve to obtain the chlorogenic acid phospholipid complex;
[0055] (2) Weighing the second portion of chlorogenic acid and the chlorogenic acid-phospholipid complex obtained in step (1), mixing them in an equal increment method, and stirring evenly to obtain a pharmaceutical composition containing chlorogenic acid.
[0056] The mass ratio of dimyristoylphosphatidylglycerol, the first chlorogenic acid, and 80% ethanol aqueous solution is 4:10:2000.
[0057] The mass ratio of the second chlorogenic acid to the chlorogenic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com