Method for synthesizing isoquinolinone compounds or pyridone compounds
A technology for isoquinolinones and compounds, applied in the field of synthesizing isoquinolinones or pyridones, achieving the effects of mild conditions, simple raw materials, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~13
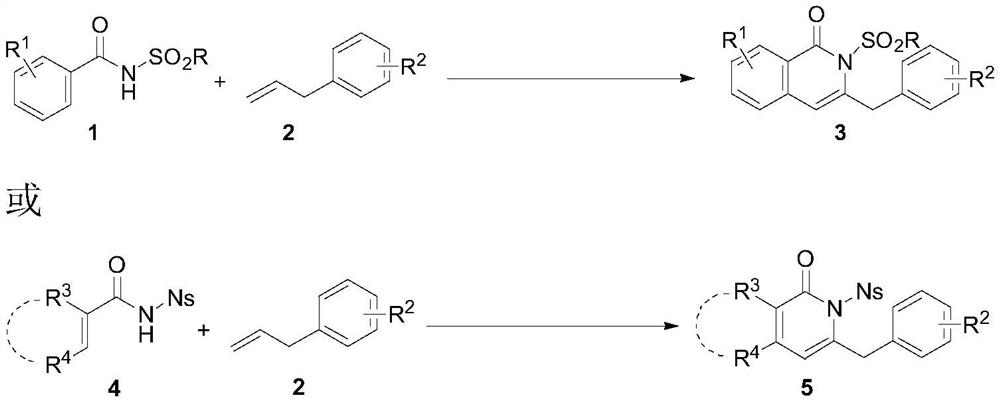
[0028] Add benzamide 1a (0.2mmol), phenylpropylene 2a (0.4mmol), catalyst (0.02mmol), cocatalyst (0.04mmol), p-xylene 2mL to a 10ml Shrek tube, and heat to react for 24 hours. Then the reaction system was cooled to room temperature, sand core filtered, washed with ethyl acetate, the solvent was concentrated, and the target product 3a was obtained by passing through the column with petroleum ether / ethyl acetate=5 / 1. The specific reaction conditions and reaction results are shown in Table 1.
[0029] The reaction formula is as follows:
[0030]
[0031] The reaction condition and reaction result of table 1 embodiment 1~13
[0032]
[0033]
[0034] a Reaction conditions: 1a (0.2mmol), 2a (0.4mmol), catalyst (0.02mmol), co-catalyst (0.04mmol), p-xylene (2ml), 110°C under air atmosphere for 24h; b Separation yield; c oxygen balloon; d 100°C.
Embodiment 14~29
[0036] Add benzamide 1 (0.2mmol), phenylpropylene 2 (0.4mmol), palladium trifluoroacetate (6.6mg, 0.02mmol), copper acetate (7.3mg, 0.04mmol), p-xylene 2mL to a 10ml Shrek tube, 110 degrees open stirring reaction for 24 hours. Then the reaction system was cooled to room temperature, filtered with sand core, washed with ethyl acetate, concentrated solvent, and passed through the column with petroleum ether / ethyl acetate=5 / 1 or 3 / 1 to obtain target products 3b~3q, the reaction conditions and results are as follows:
[0037]
Embodiment 30~38
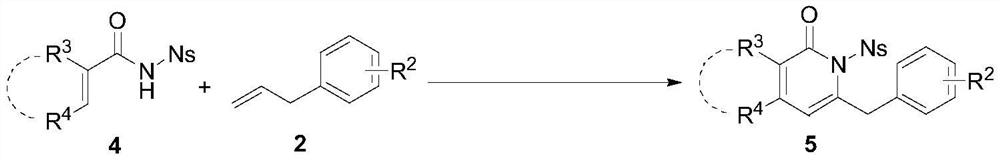
[0039] Add acrylamide 4 (0.2mmol), phenylpropylene 2 (0.4mmol), palladium trifluoroacetate (6.6mg, 0.02mmol), copper acetate (7.3mg, 0.04mmol), p-xylene 2mL, 110 The reaction was carried out under open stirring for 24 hours. Then the reaction system was cooled to room temperature, sand core filtered, washed with ethyl acetate, the solvent was concentrated, and the target products 5a-5i were obtained by passing through the column with petroleum ether / ethyl acetate=5 / 1 or 3 / 1.
[0040]
[0041] Product characterization data are characterized as follows:
[0042] Isoquinolinones:
[0043] 3-Benzyl-2-((4-nitrophenyl)sulfonyl)isoquinolin-1(2H)-one(3a).Lightyellow solid; 73.9 mg; 88% yield; R f = 0.44 (hexane / EtOAc = 5 / 1 as the eluent); mp = 193 - 194 °C; 1 H NMR (400MHz, CDCl 3 )δ8.42–8.36(m,4H),7.97(dd,J=7.8,1.4Hz,1H),7.48(td,J=7.5,1.4Hz,1H),7.45–7.40(m,2H),7.37 –7.32(m,4H),7.16(d,J=7.6Hz,1H),6.82(s,1H),4.01(s,2H); 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ163.1, 150.6, 145.3, 137...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com