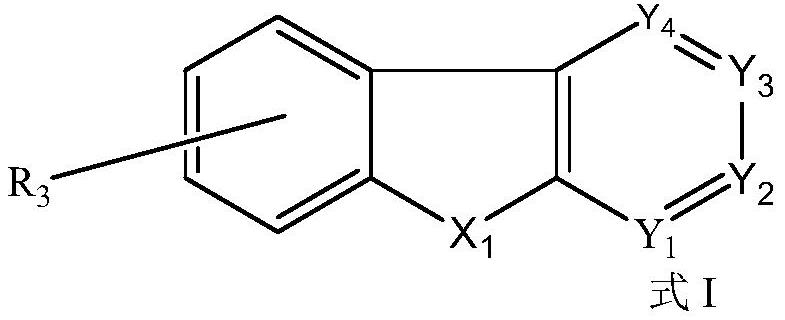
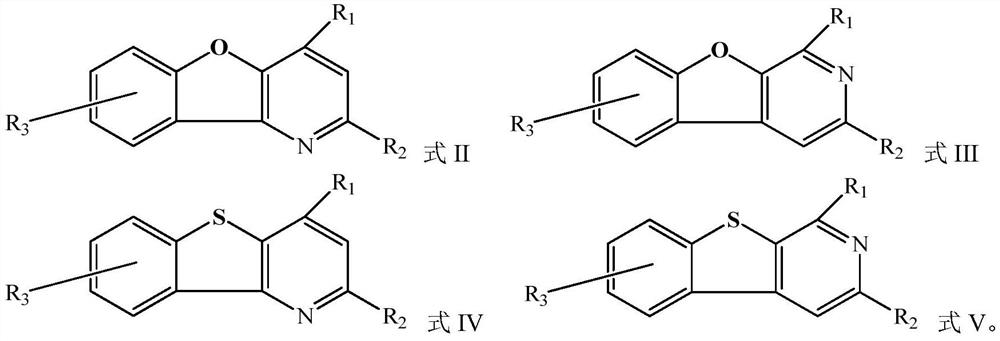
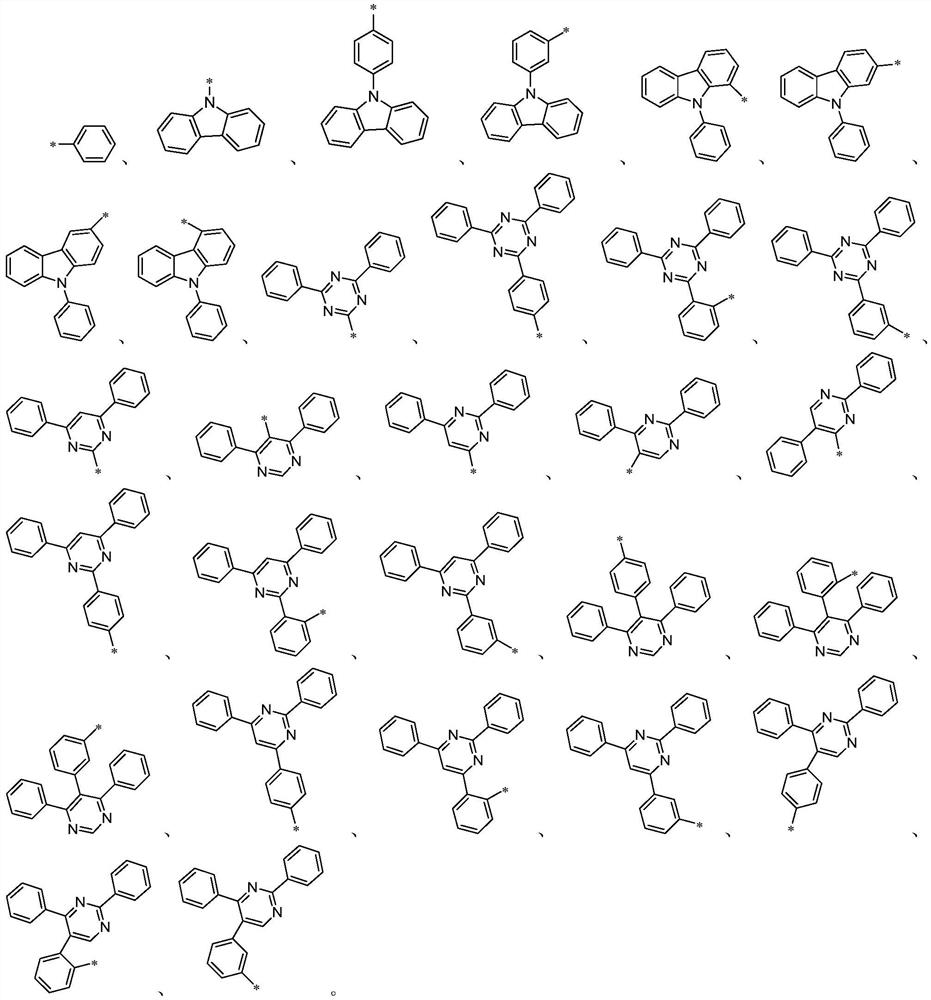
Organic compound and organic electroluminescent device
A technology of organic compounds and substituents, applied in the field of organic electroluminescent devices, can solve problems such as carrier imbalance, reduced efficiency of organic electroluminescent devices, energy loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0146]
[0147] Synthesis of Intermediate 3-1: In a 500ml three-neck flask, under nitrogen protection, sequentially add Intermediate M1 (50mmol, 14.8g), biborpinacol ester (60mmol, 15.3g), potassium acetate (0.13mol, 12.8 g), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.5mmol, 0.37g) and 1,4-dioxane solvent (160ml), heating and stirring, heating After 8 hours of reflux reaction, the reaction of the raw materials was detected by HPLC. After the reaction solution was lowered to room temperature, the reaction solution was spin-dried under reduced pressure to obtain a crude product. The crude product was dissolved in chlorobenzene solvent, heated and stirred, heated to reflux, and heated through a silica gel column for For decolorization, the filtrate was spin-dried under reduced pressure until there was a small amount of solvent, then 200 ml of ethanol was added to make a slurry, and recrystallized with toluene / ethanol to obtain a white solid (yield: 88%).
[...
preparation example 2
[0153]
[0154] Synthesis of Intermediate 11-1: The synthesis method was the same as that of Intermediate 3-1 to obtain a white solid (yield: 86%).
[0155] Synthesis of Intermediate 11-2: The synthesis method was the same as that of Intermediate 3-2 to obtain a white solid (yield: 70%).
[0156] Synthesis of Intermediate 11-3: The synthesis method was the same as that of Intermediate 3-3 to obtain a white solid (yield: 93%).
[0157] Synthesis of compound 11: the synthesis method was the same as that of compound 3 to obtain a white solid (yield: 65%).
[0158] Mass spectrum: C50H31N5O, theoretical value: 717.25, measured value: 717.2. 1H-NMR (400MHz, CDCl3) (ppm) δ=7.10~7.17 (2H, m), 7.33~7.38 (1H, m), 7.42~7.49 (10H , m), 7.50~7.52(1H, m), 7.53~7.61(4H, m), 7.63~7.68(2H, m), 7.80~7.85(1H, m), 8.12~8.16(1H, m), 8.22 ~8.26 (3H, m), 8.28 ~ 8.35 (4H, m), 8.38 ~ 8.40 (1H, m), 8.53 ~ 8.55 (1H, m).
preparation example 3
[0160]
[0161] Synthesis of Intermediate 18-1: The synthesis method was the same as that of Intermediate 3-1 to obtain a white solid (yield: 83%).
[0162] Synthesis of Intermediate 18-2: The synthesis method was the same as that of Intermediate 3-2 to obtain a white solid (yield: 72%).
[0163] Synthesis of Intermediate 18-3: The synthesis method was the same as that of Intermediate 3-3 to obtain a white solid (yield: 89%).
[0164] Synthesis of Compound 18: The synthesis method was the same as that of Compound 3 to obtain a white solid (yield: 66%).
[0165]Mass spectrum: C50H31N5O, theoretical value: 717.25, measured value: 717.2. 1H-NMR (400MHz, CDCl3) (ppm) δ=7.16~7.21 (2H, m), 7.38~7.44 (1H, m), 7.48~7.53 (11H , m), 7.55~7.61(5H, m), 7.61~7.63(1H, m), 7.69~7.73(1H, d), 7.85~7.89(1H, m), 8.17~8.21(1H, m), 8.27 ~8.31 (2H, m), 8.34~8.39 (5H, m), 8.44~8.44 (1H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com