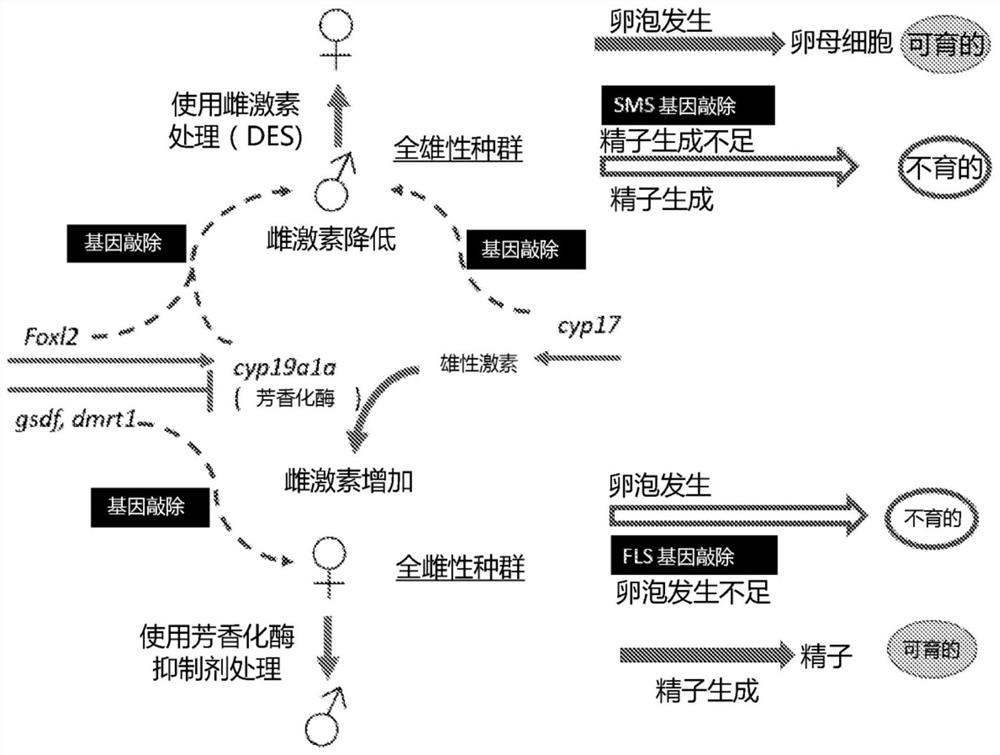
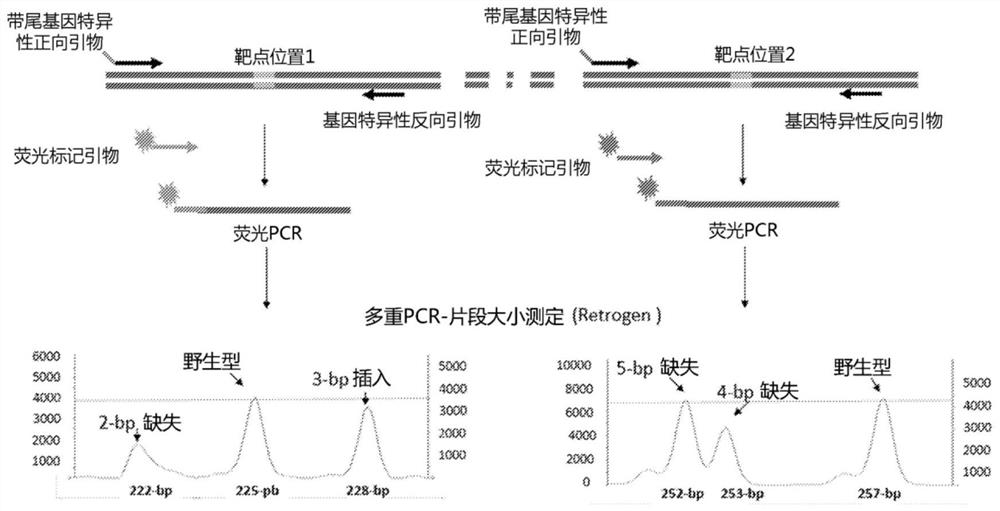
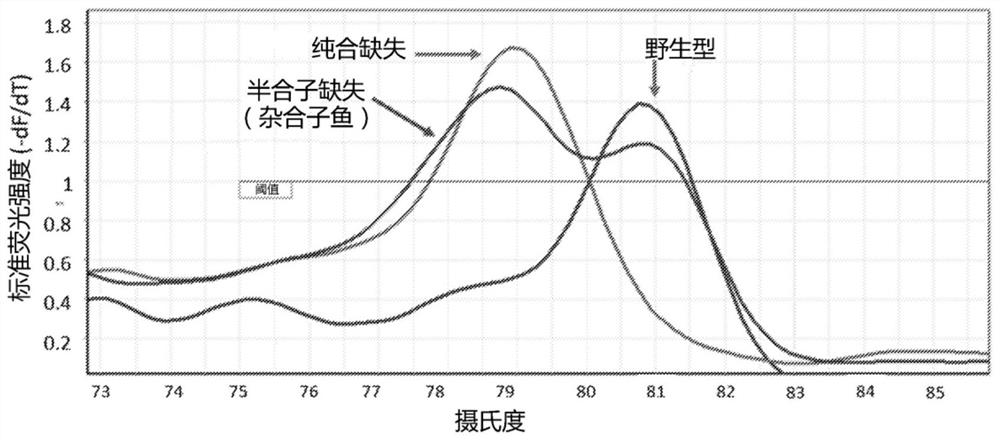
A method of generating sterile and monosex progeny
A fertile, female technology for the production of sterile and parthenogenetic progeny that addresses increased operating costs, increased disease susceptibility, and increased mortality in sterile organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 - Materials and methods
[0107] Animals used and ethics statement: All experiments complied with US regulations, ensuring that animal welfare and husbandry procedures were performed in accordance with the IACUC-approved animal protocol CAT-004. The tilapia (Oreochromis niloticus) strain used in this study was derived from a Brazilian strain obtained from a commercial producer in the United States.
[0108] Nuclease Generation and Its Strategy: Generation of F0 mutants: homologous gene lines of cyp17, Cyp19a1a, Tjp1a, Csnk2a2, Hiat1, Smap2, Gopc, Gsdf, Dmrt1, FSHR and vitellogenin genes (VtgAa and VtgAb) of tilapia were obtained from genome databases by computer Identification.
[0109] To generate DNA double-strand breaks (DSBs) at specific genomic loci, we used engineered nucleases. In most applications, a single DSB is generated in the absence of a repair template, resulting in activation of the non-homologous end-joining (NHEJ) repair pathway. NHEJ ...
Embodiment 2
[0126] Example 2 - Using gene editing tools to induce biallelic knockout in the F0 generation of tilapia
[0127] We independently targeted two genes involved in pigmentation, the gene encoding tyrosinase (tyr) [2] and the mitochondrial inner membrane protein MpV17 (MpV17) (Krauss, Astrinides et al. 2013) [8] . We found that 50% and 46% of the injected embryos showed hypermutation at the tyr and mpv17 loci, respectively ( Figure 4 ). Loss of the cell-function allele autonomously leads to the embryonic body ( Figure 4 B) and retinal pigment epithelium ( Figure 4 The achromatic cell mass in C) produces embryonic phenotypes ranging from complete loss of melanin to partial loss and iris pigmentation that are easily differentiated from wild-type phenotypes ( Figure 4 A and C). Embryos showing complete lack of pigmentation (10-30% of treated fish) were reared to 3 months of age, all lacking wild type tyr and mpv17 sequences. These fish display transparent and albino phenot...
Embodiment 3
[0128] Example 3 - Multigene Targeting in Tilapia
[0129] We tested whether multiple loci in the tilapia genome could be targeted simultaneously and whether mutagenesis efficiency measured at one locus could predict mutations at other loci. To test our hypothesis, we co-targeted tyr and dead-end 1 (dnd). Dnd is a PGC-specific RNA-binding protein (RBP) that maintains germ cell fate and migration ability [3]. After injection of programmed nuclease, we found that mutations in two gene targets, Tyr and Dnd, were highly correlated. Approximately 95% of abino(tyr) mutants also carry mutations at the dnd locus, demonstrating the suitability of pigmentation defects as selectable markers ( Figure 5 A). After further analysis of the gonads of 10 albino fish, 6 had translucent agenic testes ( Figure 5 B). Vasa, a germ cell-specific marker strongly expressed in wild-type testis, was not significantly expressed in dnd mutant testis. This result suggests that expression of zygotic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com