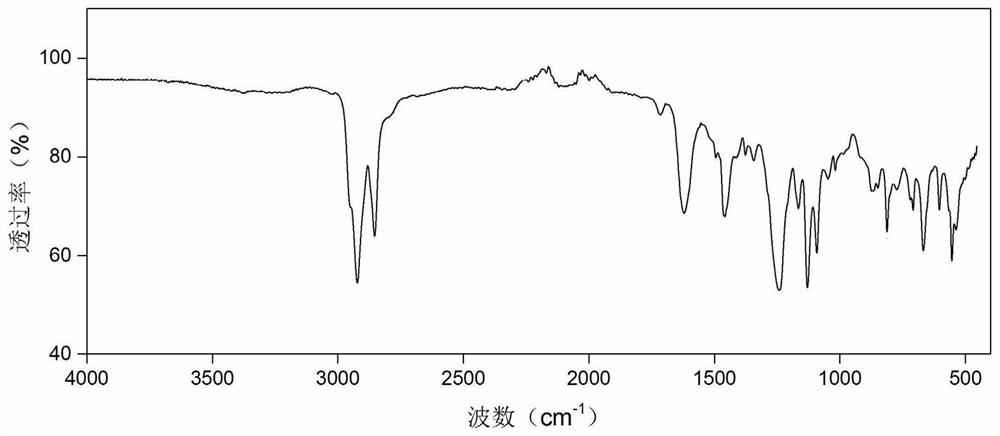
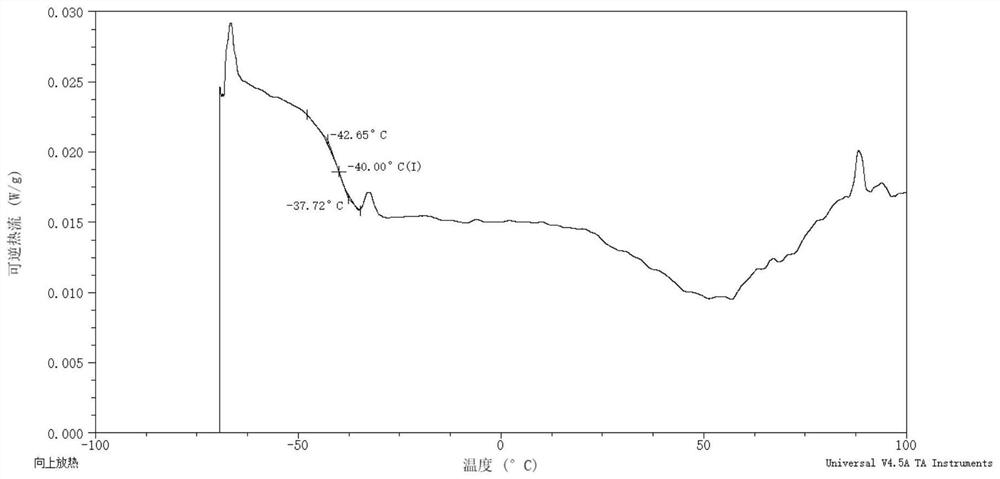
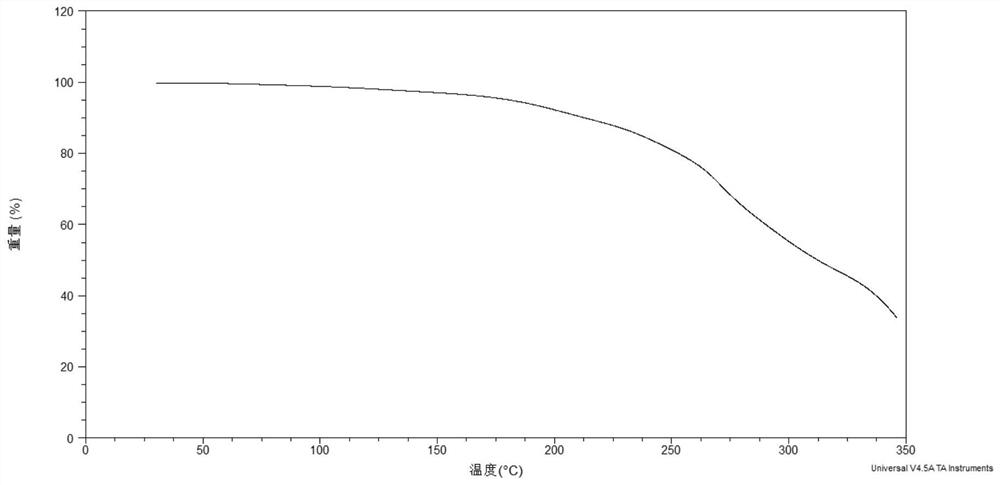
Gliclazide ionic liquid as well as preparation method and application thereof
A technology of ionic liquid and gliclazide, which is applied in the field of medicine and can solve problems such as gastrointestinal disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1. Preparation of Tetrabutylphosphonium-Glicazide Ionic Liquid
[0090] 200 mg (0.62 mmol) of gliclazide was suspended in 10 mL of ethyl acetate, an equimolar amount of tetrabutylphosphonium hydroxide (40% aqueous solution) was added, and stirred at room temperature overnight until a clear solution was obtained. The solvent was removed by rotary evaporation at 60°C, and vacuum-dried at 40°C for 72 hours to obtain tetrabutylphosphonium-gliclazide ionic liquid.
[0091] Proton NMR spectrum of tetrabutylphosphonium-gliclazide ionic liquid:
[0092] 1H NMR (500MHz, Chloroform-d) δ7.78(d, J=8.0Hz, 2H), 7.09(d, J=7.9Hz, 2H), 3.01(t, J=8.1Hz, 2H), 2.51–2.39 (m,2H),2.29(s,3H),2.24–2.06(m,8H),1.93(s,2H),1.60–1.47(m,4H),1.42(dh,J=7.4,3.6Hz,16H ),1.36–1.27(m,2H),0.96–0.81(m,12H).
[0093] The imino group next to the sulfonyl group in gliclazide is the most acidic, and it is easy to lose a proton, making it ionized. The chemical shift of the hydrogen at this position...
Embodiment 2
[0097] Embodiment 2. Tetrabutylphosphonium-gliclazide ionic liquid and gliclazide solubility comparison
[0098] Excess tetrabutylphosphonium-gliclazide ionic liquid and gliclazide were weighed and suspended in pure water. After shaking at 37°C for 24 hours, the solubility was tested by high-performance liquid chromatography. The test results were as follows:
[0099] Table 1
[0100] compound Solubility (μg / mL) tetrabutylphosphonium-gliclazide 2153.19 Grecht 43.52
[0101] From the above results, it can be concluded that the solubility of tetrabutylphosphonium-gliclazide ionic liquid in pure water is significantly higher than that of gliclazide.
Embodiment 3
[0102] Example 3. Tetrabutylphosphonium-gliclazide ionic liquid and comparison of the in vitro release of gliclazide
[0103] Release tetrabutylphosphonium-gliclazide ionic liquid and gliclazide prepared in Example 1 according to the method provided in the first method (basket method) in the general rule 0931 dissolution and release assay of Chinese Pharmacopoeia 2015 edition degree measurement. The specific method is as follows:
[0104] Test with simulated gastric juice or simulated intestinal juice (simulated gastric juice: take 2.0g sodium chloride and 3.2g pepsin, add 7.0ml hydrochloric acid and water to dissolve to 1000ml, and the pH value of the solution should be 1.2. Simulated intestinal juice: take phosphoric acid Dihydropotassium 6.8g, add 250ml of water to dissolve, add 77ml of 0.2mol / L sodium hydroxide solution and 500ml of water, add 10g of trypsin to dissolve, then use 0.2mol / L sodium hydroxide solution or 0.2mol / L hydrochloric acid solution Adjust the pH valu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap