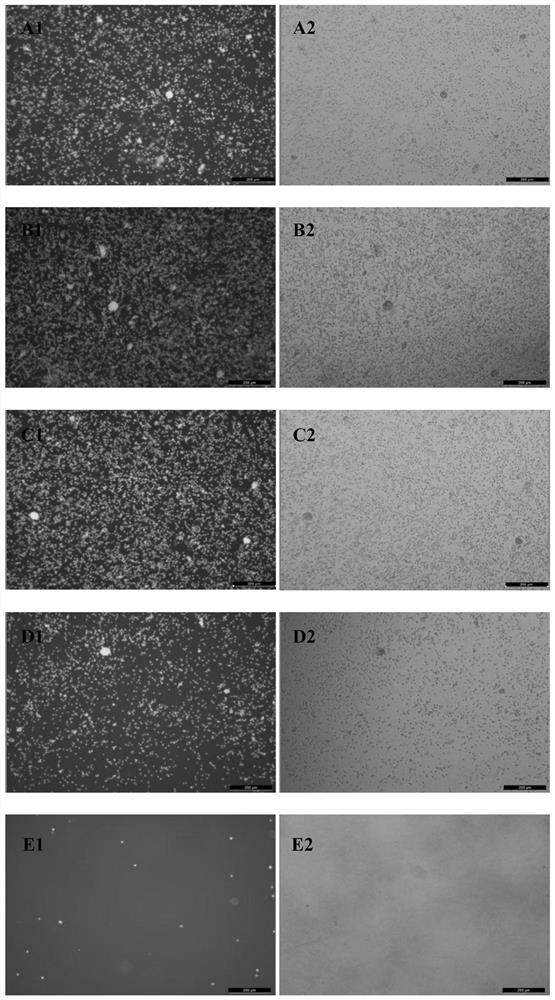
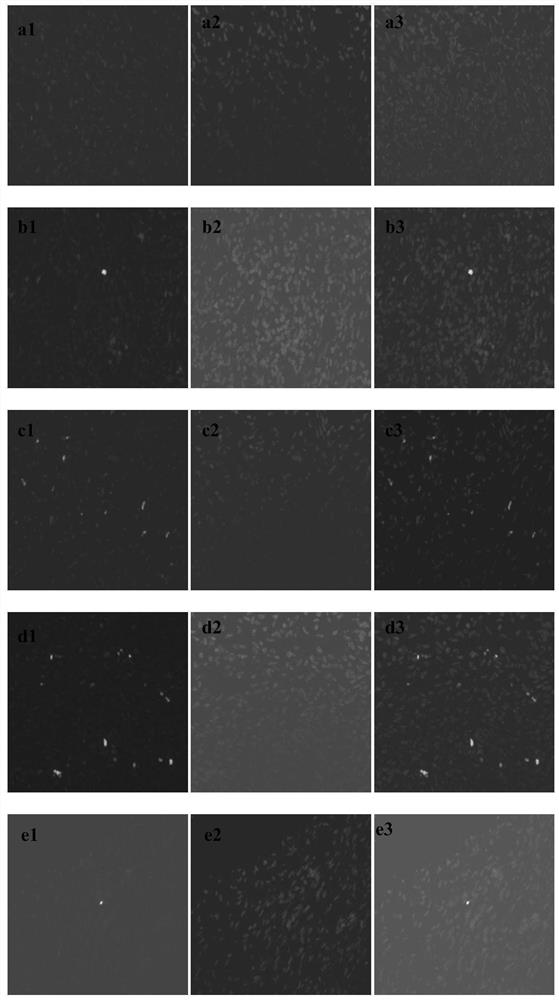
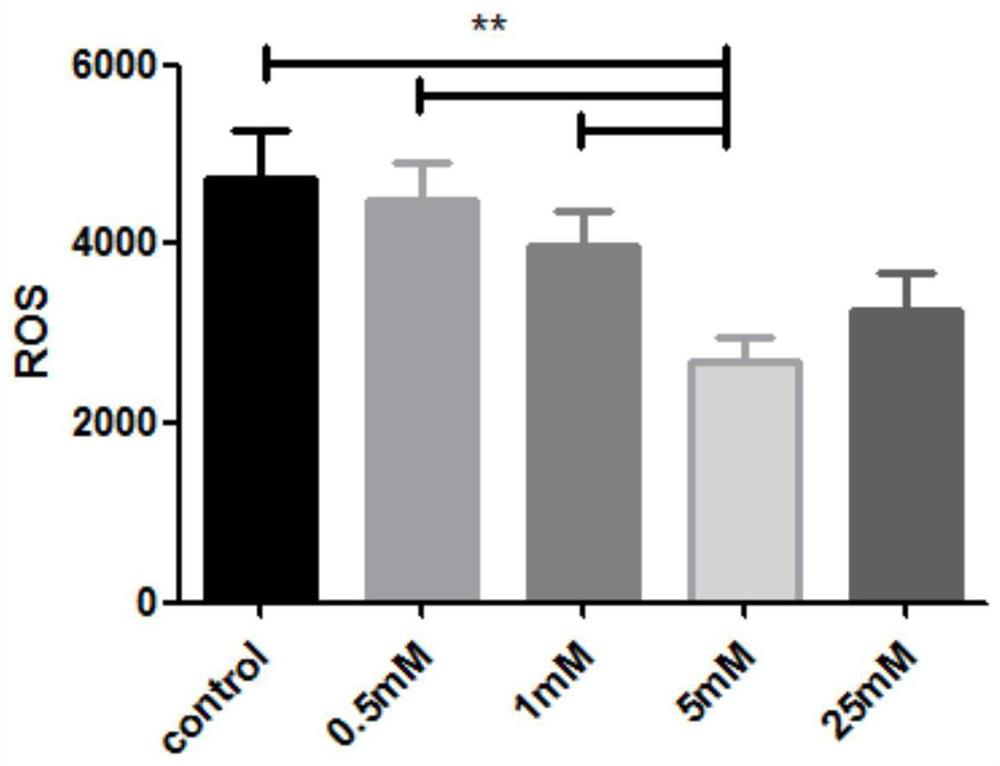
Cryopreservation resuscitation fluid for ovarian tissue
一种卵巢组织、复苏液的技术,应用在卵巢组织的冻存复苏液领域,达到好冷冻保存生殖力、降低副反应、稳定蛋白质的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0099] Embodiment 1-6 Preparation of Ovarian Tissue Cryopreservation Solution
[0100] Take the preparation of 100ml cryopreservation solution as an example: Measure LeibovitzL-15 medium, DMSO, HSA, and SSS into the reagent bottle according to the amount of each component in Table 1, shake gently to mix, and use a 0.22um filter to Sterilize by filtration, filter into an autoclaved reagent bottle, store at 4°C for later use, or freeze at -20°C, and move to a 4°C refrigerator for later use.
[0101] Table 1 Components of Cryopreservation Solution 1
[0102]
Embodiment 7-17
[0103] Example 7-17 Preparation of Ovarian Tissue Cryopreservation Solution
[0104] Take the preparation of 100ml cryopreservation solution as an example: Measure LeibovitzL-15 medium, DMSO, HSA, and SSS into the reagent bottle according to the amount of each component in Table 2, shake gently to mix, and then add the corresponding amount taurine, nitrogen acetylcysteine, vitamin E, vitamin C and heparin. After the solid is completely dissolved, use a 0.22um filter to filter and sterilize, filter into an autoclaved reagent bottle, store at 4°C for later use, or freeze at -20°C, and move to a 4°C refrigerator for later use.
[0105] Table 2 Components of cryoprotective solution 2
[0106]
[0107]
Embodiment 18-20
[0108] The preparation of embodiment 18-20 ovarian tissue resuscitating liquid
[0109] Take the preparation of 100ml cryopreservation solution as an example: Measure LeibovitzL-15 medium, DMSO, HSA, and SSS into the reagent bottle according to the amount of each component in Table 2, shake gently to mix, or add the corresponding Amount of taurine, nitrogen acetylcysteine, vitamin E, vitamin C. After the solid is completely dissolved, use a 0.22um filter to filter and sterilize, filter into an autoclaved reagent bottle, store at 4°C for later use, or freeze at -20°C, and move to a 4°C refrigerator for later use.
[0110] Table 3 Composition of resuscitation solution
[0111]
[0112]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com