Dihydropyridine calcium antagonist salt composition as well as preparation method and application thereof
A technology of calcium antagonist and dihydropyridine, which is applied in the field of levamlodipine composition and its preparation, and dihydropyridine calcium antagonist drug, which can solve the problems of low melting temperature, poor solubility, and easy bonding, etc. Achieve the effects of high process temperature, high dissolution rate and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Levoamlodipine: HPMCP salt composition preparation:
[0037] Under magnetic stirring, add 0.4g levamlodipine and 1.6g HPMCP into ethanol / dichloromethane 25mL (1:1 volume ratio) mixed solvent, make it dissolve to form a solution, basic levamlodipine and acidic polymer Ionic bonds are formed between HPMCPs. Then use the miniature spray drier B290 of inert cycle B295 ( Labortechnik AG, Switzerland) spray drying, a high-performance cyclone separator was used for separation, and a 50 mL blue cap flask could be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 1.
[0038] Table 1
[0039] parameter Settings Suction 40kg / h Inlet temperature 80℃ output temperature 60℃ Injection rate 5mL / min Atomizing airflow 0.5kg / h Inert loop cooling temperature -20℃
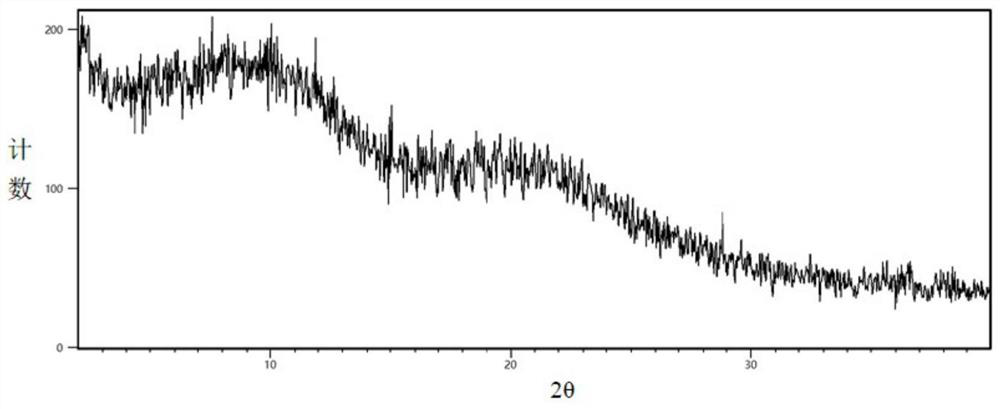
[0040] The levamlodipine that contains 20% levamlodipine (mass ratio) obtained aft...
Embodiment 2
[0042] Levoamlodipine: HPMCAS salt composition preparation:
[0043] Under magnetic stirring, add 0.4g levamlodipine and 1.6g HPMCAS into ethanol / dichloromethane 25mL (1:1 volume ratio) mixed solvent, make it dissolve to form a solution, basic levamlodipine and acidic polymer Ionic bonds are formed between HPMCAS. Then use the miniature spray drier B290 of inert cycle B295 ( Labortechnik AG, Switzerland) spray drying, a high-performance cyclone separator was used for separation, and a 50 mL blue cap flask could be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 2.
[0044] Table 2
[0045] parameter Settings Suction 40kg / h Inlet temperature 70℃ output temperature 54℃ Injection rate 5mL / min Atomizing airflow 0.5kg / h Inert loop cooling temperature -20℃
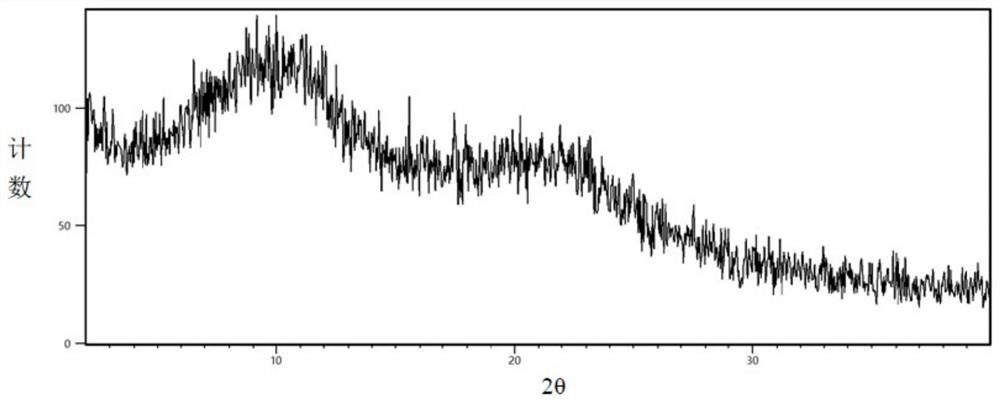
[0046]The levamlodipine containing 20% levamlodipine (mass ratio) obtained aft...
Embodiment 3
[0047] Embodiment 3: preparation of levamlodipine besylate
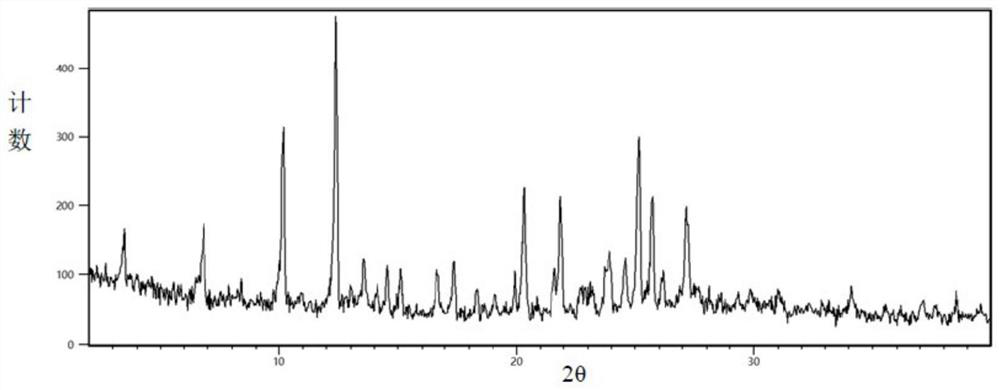
[0048] Take 1.3g of levoamlodipine, add 10mL of methanol to dissolve completely, add 1.5mL of 40% mass fraction of benzenesulfonic acid aqueous solution and stir for 5min, add 50mL of water and continue stirring for 20min to form a large amount of precipitate, filter, wash with 30mL of water, and then transfer to vacuum drying Vacuum drying in oven at 40°C for 16h. The obtained solid powder is levamlodipine besylate 2.5 hydrate. Detecting the PXRD pattern is consistent with CN11689894A, such as image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com