(R)-CuTAPBP-COF polymer as well as preparation method and application thereof
A polymer, -BIONLPA-DA technology, applied in the direction of organic chemical methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem that homogeneous catalysts are difficult to separate, difficult to recycle, etc. problem, to achieve high yield and stereoselectivity, easy recovery, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051]Further, the preparation method includes reacting Cu-TAPP and (R) -BionLPa-DA to the mixed solution of ethanol, average toluene and dilute acetic acid, i.e., the polymer (r) -CutApbp-COF.
[0052] Preferably, the reaction temperature is 110-120 ° C.
[0053] Preferably, the reaction time is 3-3.5d.
[0054] Preferably, the molar ratio of Cu-TAPP and (R) -BionLPa-Da is 1: 2.
[0055] Preferably, the volume ratio of the ethanol, average toluene and dilute acetic acid is 17: 3: 2.
[0056] Further preferred, the preparation method also includes: After the reaction, the solid portion is collected by ethanol, which is obtained by ethanol, i.e., the polymer (R) -CutApBP-COF.
[0057] In one or more embodiments of the invention, the synthesis method of the (R) -bionlpa-DA is:
[0058] Under nitrogen, weigh the amount of (R) -BionLDH-DA, add anhydrous pyridine, and then dropped to the reaction system plus POCl. 3 , Mixed, resulting in (r) -bionlpa-da.
[0059] Further, (R) -BionLDH-...
Embodiment 1
[0092] Example 1: (r) Synthesis of -BionLPa-DA
[0093] (R) -1, 1'-joint-2-naphthol (10.0 mmol, 2.86 g) was placed in a 100 ml of three flasks, dichloromethane (20 mL), cooled to 0 ° C, slowly dropped under stirring. Liquid bride (25.2 mmol, 4.00 g), reacted at 0 ° C for 24 h, add NA 2 S 2 O 3 (7.4 mmol, 1.20 g) of aqueous solution was quenched, and stirring was continued for 2 h. After the reaction is completed, the reaction liquid becomes pale yellow by orange. The reaction solution was filtered to the liquid filter into the separatory funnel, and the organic phase was washed three times with a saturated NaCl solution, and the organic phase was stirred and evaporated in vacuo to give a light yellow solid (R) -db.
[0094] Weigh (R) -DB (10.0 mmol, 4.44g), no water K 2 CO 3 (40.0 mmol, 5.50 g), bromoethane (60.0 mmol, 7.00 g) was placed in a 100 mL of two flasks, and acetone (40 ml) was added, and the heat was refluxed for 48 h. After the reaction was completed, cooled to room te...
Embodiment 2
[0100] Example 2: (r) Synthesis of -Cutapbp-Cof
[0101] Cu-TAPP (0.05 mmol, 36.60 mg), (r) -bionlPa-DA (0.1 mmol, 61.60 mg) was placed in a 10 ml pressure resistant tube, adding acetic acid (9M, 0.2 mL), ethanol / average toluene ( 5: 1, 2 mL) Mixed solvent, ultrasonic 10min makes mixing uniform. The liquid nitrogen was frozen three times, and then heated at 120 ° C for 72 h. After the reaction was completed, then cooled to room temperature, and left 48 h. The solid was centrifuged, washed with ethanol, and the purple black product (R) -CutApbp-Cof was obtained with ethanol.
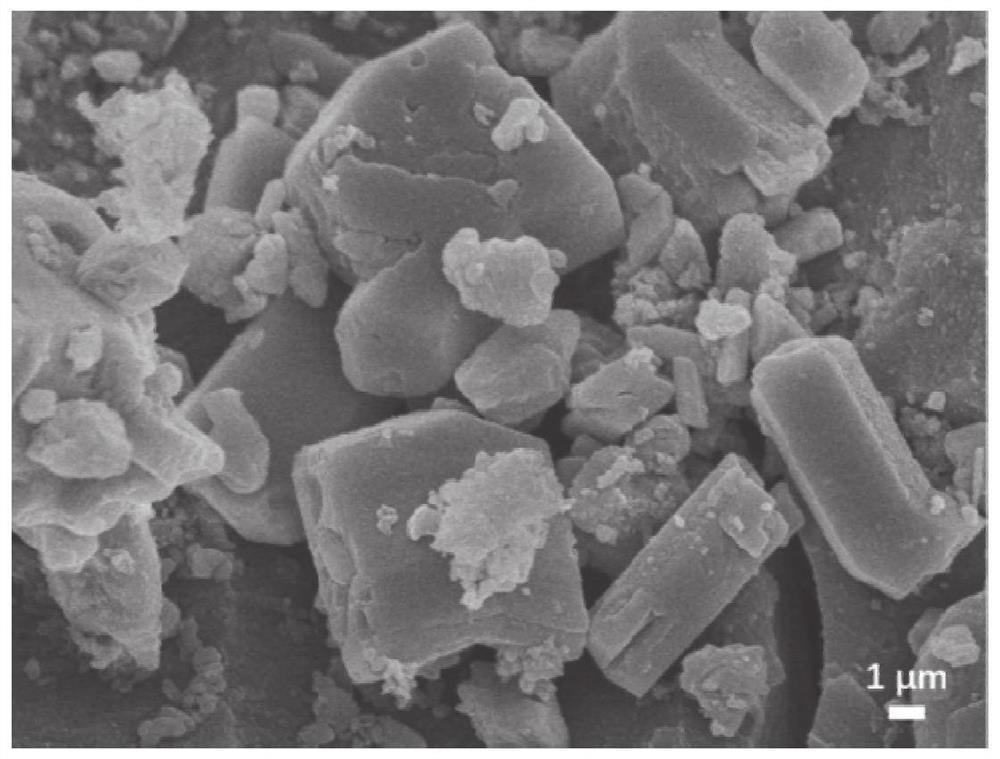
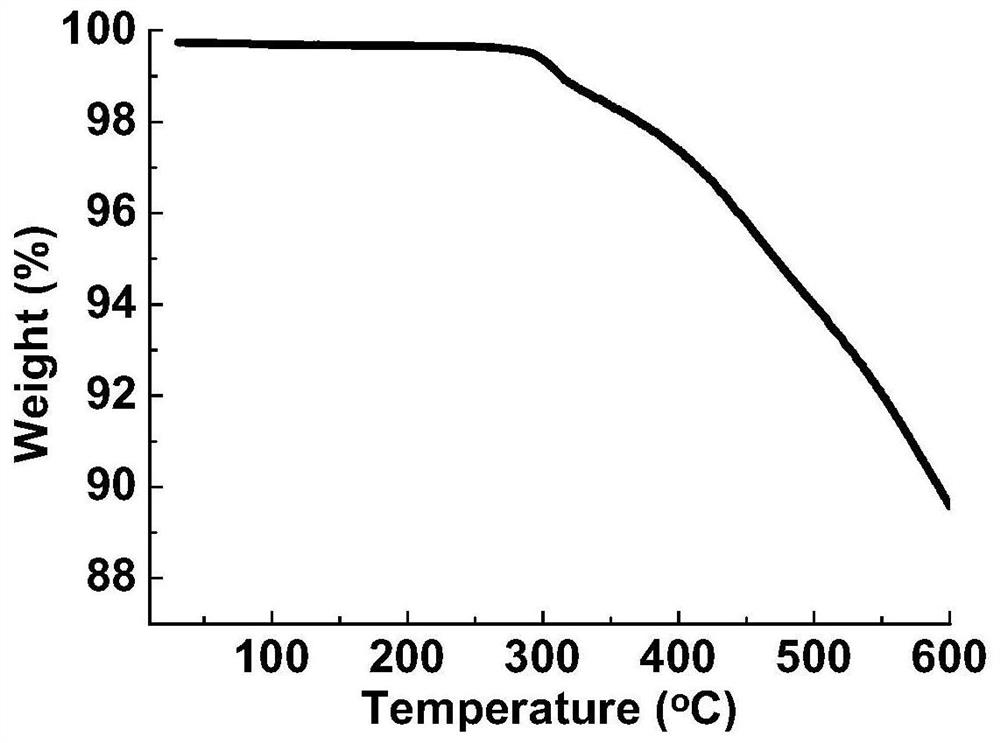
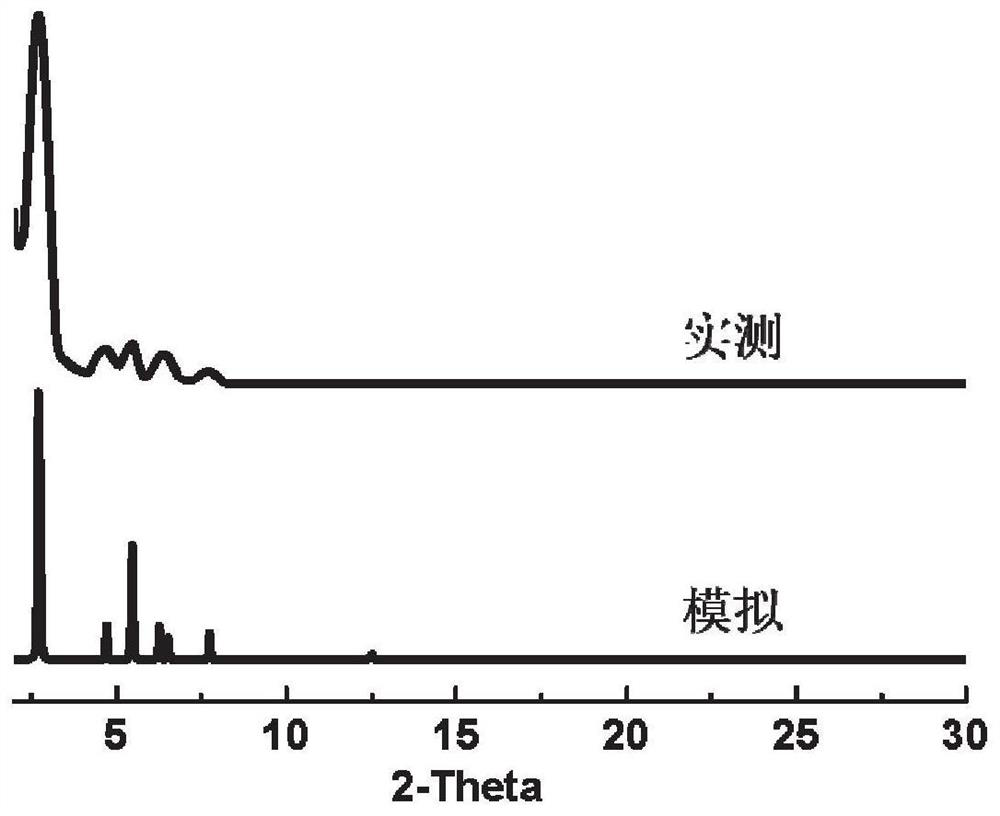
[0102] This embodiment is passed by scanning electron microscopy, thermal weight, PXRD, solid nuclear magnetic, N 2 Adsorption characterized the compound, the results were respectively figure 1 , 2 , 3, 4 and 5, by figure 1 and Figure 5 The polymer can be confirmed that the polymer has a pore structure, the heat weight of the catalyst (R) -CutApbp-COF ( figure 2 ), PXRD ( image 3 ), Solid nuclear magnetic (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com