A kind of antifungal antibacterial peptide gl4w and its preparation method and application
An antimicrobial peptide and fungal technology, applied in the biological field, can solve problems such as broad antibacterial spectrum, secondary infection, and damage to the balance of the body's flora, and achieve high application value and high cell selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Design of Antimicrobial Peptides
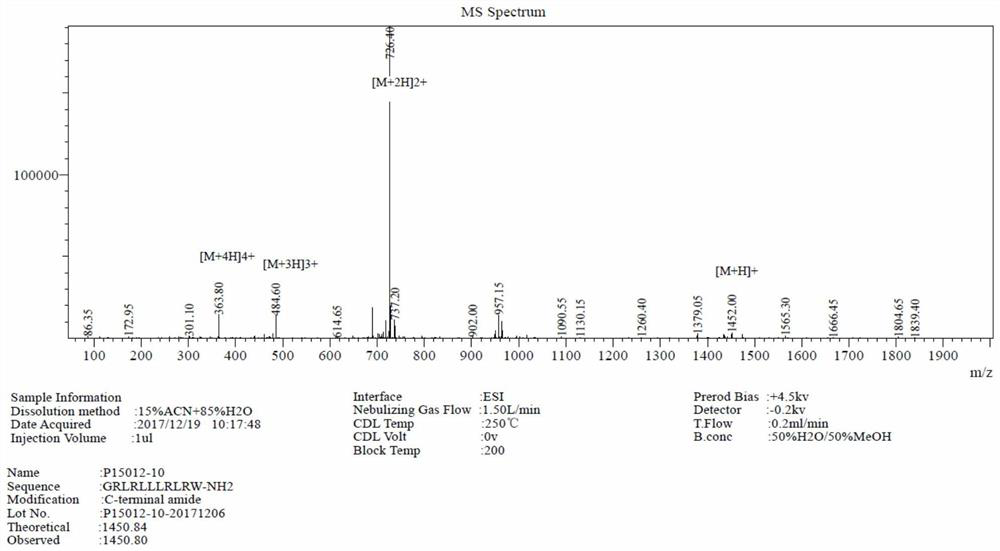
[0017] L4 (RLRLLRLR) designed from the α-helical peptide sequence template Rn(XRXXXRX)Rn (n=1,2; X represents L) is the active center, with glycine forming a cap structure at its N-terminus and adding color at its C-terminus A fungicidal antibacterial peptide was designed by using amino acid to form a tail anchor structure, named GL4W. The amino acid sequence of GL4W is shown in Table 1.
[0018] Table 1 Amino acid sequence of GL4W
[0019]
[0020] The charge number of GL4W is +5, and the average hydrophobic value is 0.610 respectively. The carboxyl terminus of GL4W was amidated to add a positive charge and increase the stability of the peptide.
Embodiment 2
[0022] GL4W was synthesized by solid-phase chemical synthesis.
[0023] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed Resin for chain protection.
[0024] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate with TFA (trifluoroacetic acid), mix the washing liquid with the above filtrate, concentrate with a rotary evaporator, and then add 10 time...
Embodiment 3
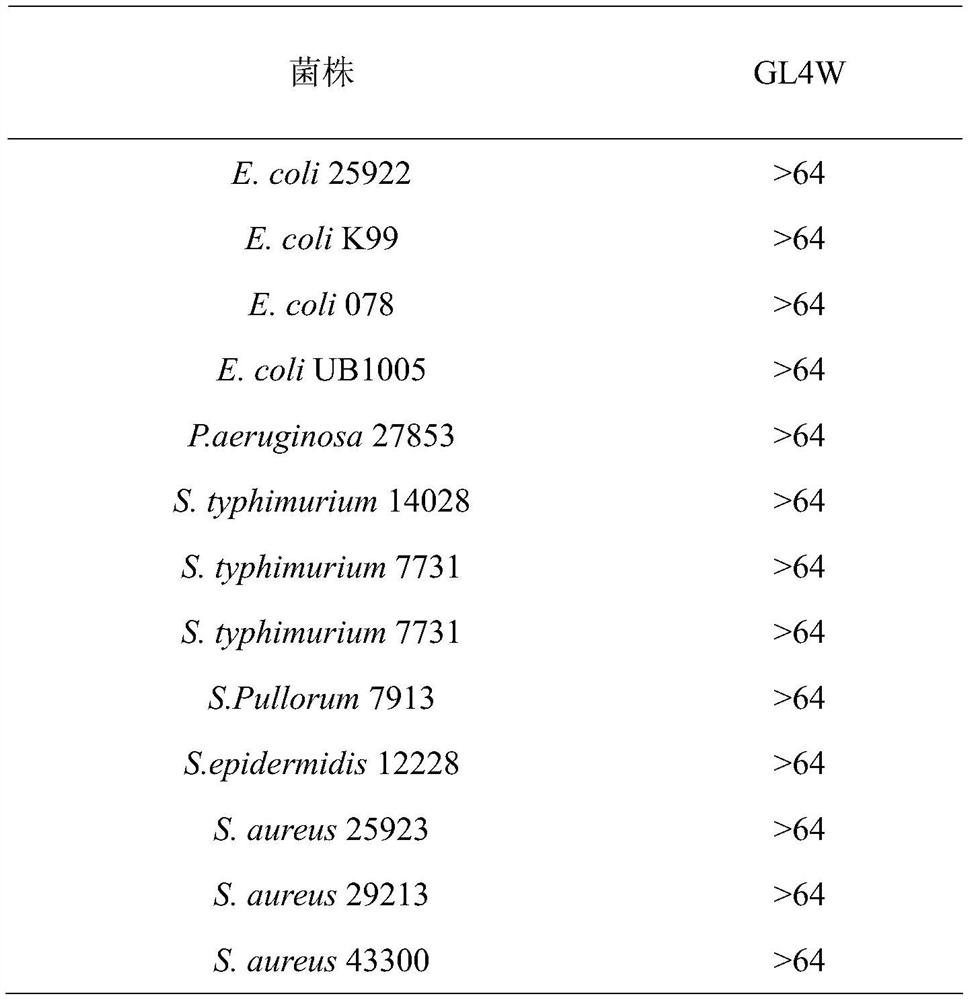
[0028] 1. Determination of GL4W antibacterial activity: This experiment refers to the method recommended by the National Committee for Clinical Laboratory Standards (NCCLS) to determine the minimum inhibitory concentration (Minimal inhibitory concentration, MIC) and the minimum bactericidal concentration ( Minimumbactericidal concentration, MBC), and make appropriate improvements according to the cationic characteristics of antimicrobial peptides, the specific steps are as follows:
[0029] (1) Bacteria preparation: Streak inoculation of strains frozen at -20°C on MHA solid medium, and culture overnight at 37°C. Then pick a single colony and inoculate it in MHB, culture at 220rpm, 37°C until the logarithmic growth phase, adjust its concentration to OD with MHB 600nm =0.1, and finally further diluted 1000 times with MHB to (0.5-1)×10 5 CFU / mL.
[0030] (2) Dilution of polypeptide: Add 95 μL of 0.2% BSA diluent to row A of the 96-well plate, and add 50 μL of 0.2% BSA diluent t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com