Anti-AFP nanometer antibody 2F5 and application thereof
A nanobody and antibody technology, applied in the field of immunology, can solve the problems of poor tumor permeability and low targeting effect, and achieve excellent detection performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
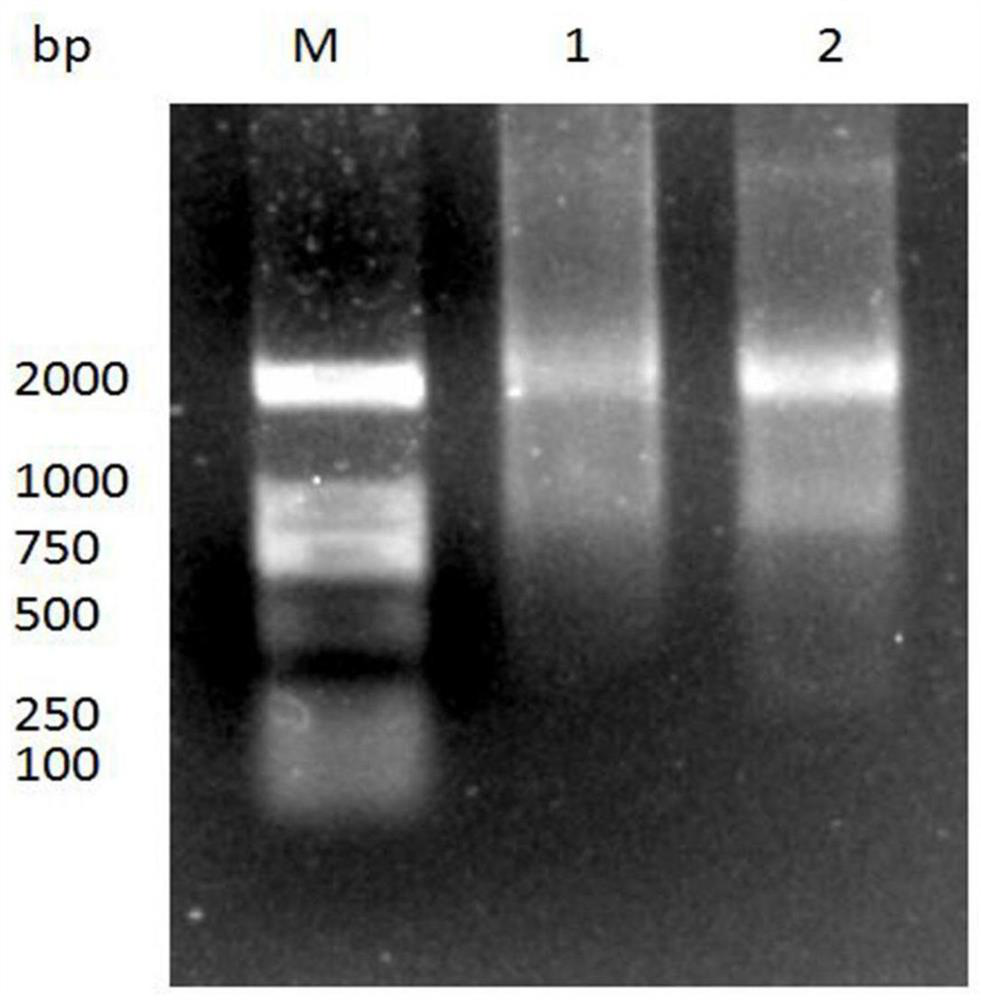
[0030] Example 1. Construction and screening of Nanobody phage display library
[0031] 1.1 Immunity of alpacas
[0032] A healthy adult alpaca was selected, and the recombinant AFP antigen (Aibisin Biological, product number Abt-P-208, GenbankID: 174, https: / / www.ncbi.nlm.nih.gov / gene / 174) and Freund's The adjuvant was mixed at a ratio of 1:1, and the alpaca was immunized with 6-7 μg / kg subcutaneously at multiple points on the back, and immunized four times with an interval of 2 weeks. Afterwards, the peripheral blood of the alpaca was collected for the construction of a phage display library.
[0033] 1.2 Isolation of camel-derived lymphocytes
[0034] Lymphocytes were separated from the collected alpaca peripheral blood using the Camel Peripheral Blood Lymphocyte Separation Solution Kit (Tianjin Haoyang Company, Cat. No. LTS1076). 7 Add 1ml RNA isolation reagent to each living cell, take 1ml for RNA extraction, and store the rest at -80°C.
[0035] 1.3 Total RNA extract...
Embodiment 2
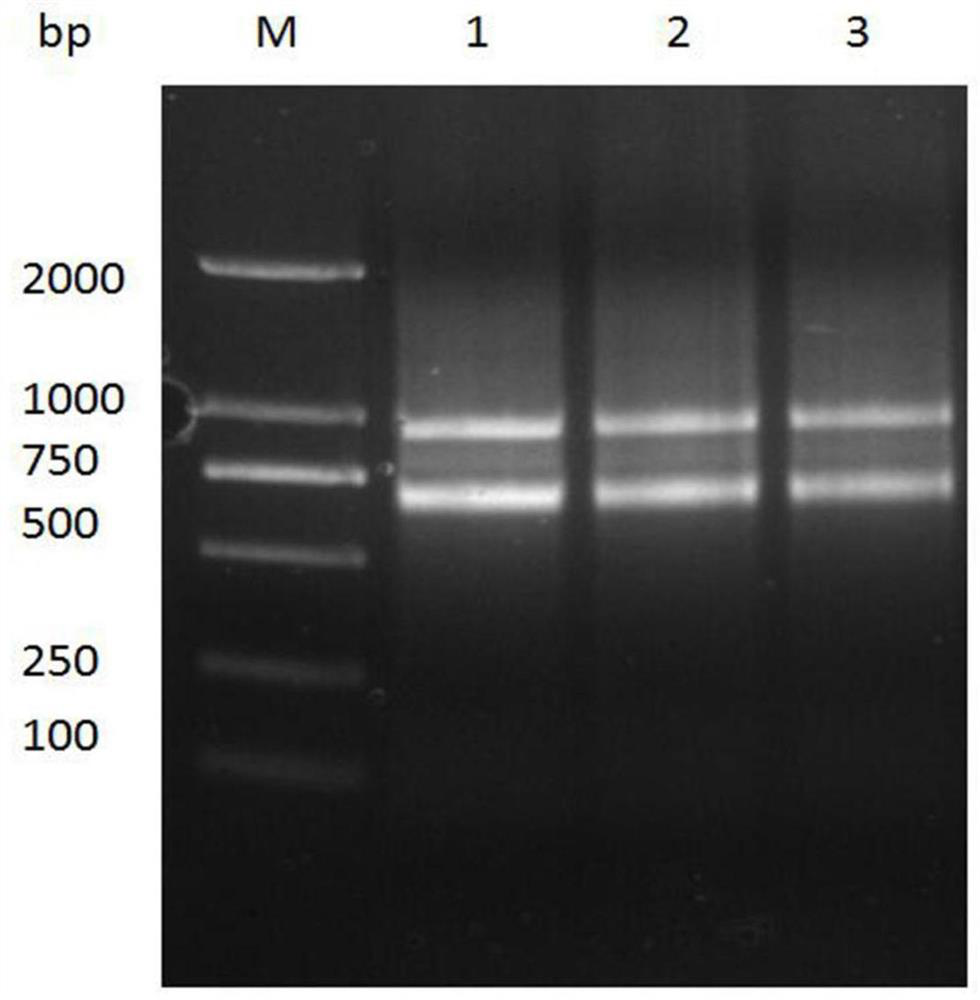
[0063] Example 2. Preparation of Nanobody 2F5
[0064] 2.1 Amplification of original nanobody strain TG1 and transformation of nanobody recombinant plasmid into Escherichia coli BL21(DE3)
[0065] Inoculate 5 ml of fresh LB-A medium at a ratio of 1:1000 to inoculate the original strain TG1 Glycerobacterium containing Nanobody nucleic acid, and culture overnight at 37°C and 200 rpm. The next day, plasmids were extracted using the Plasmid mini kit (OMEGA) according to the instructions. After verification, transform 1 μl of the above plasmid into 100 μl competent cells, mix gently, place on ice for 30 minutes, heat shock in a 42°C water bath for 90 seconds, and cool in an ice bath for 3 minutes. Add 600 μl LB medium to the centrifuge tube, shake and incubate at 37°C for 60 minutes. Take 100 μl of the supernatant, spread it on the LB-A plate with a triangular spreader, and culture it upside down at 37°C overnight.
[0066] 2.2 Induced expression of nanobodies
[0067] The abov...
Embodiment 3
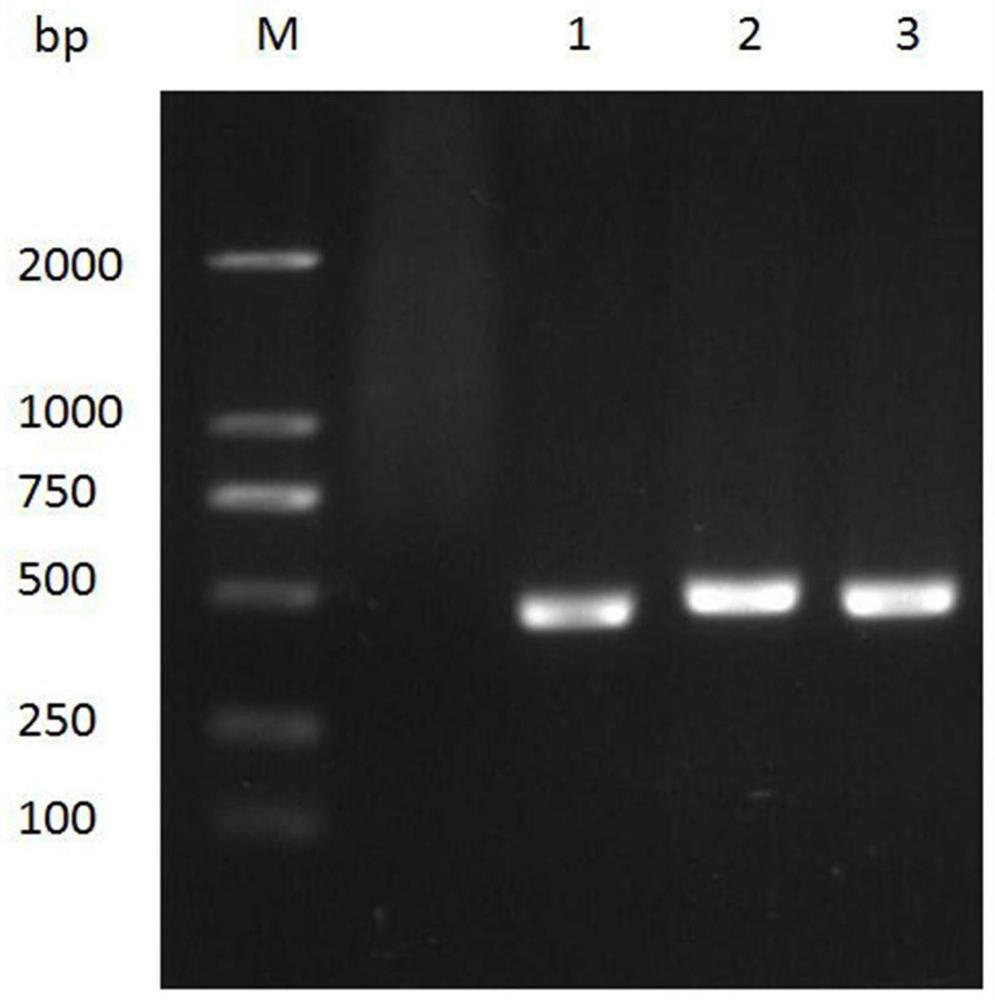
[0070] Example 3. Determination of the affinity and activity of nanobodies and antigens
[0071] 3.1 Chip antigen coupling
[0072] The antigen was formulated into a 20 μg / ml working solution with different pH sodium acetate buffers (pH 5.5, pH 5.0, pH 4.5, pH 4.0), and a 50 mM NaOH regeneration solution was prepared at the same time, and the Biacore T100 protein interaction analysis system instrument was used to The template method is used to analyze the electrostatic binding between the antigen and the surface of the chip (GE company) under different pH conditions, and the signal increase amount reaches 5 times RL as the standard, select the most suitable neutral pH system and adjust it as needed The antigen concentration was used as the condition during coupling. The chip was coupled according to the template method that comes with the instrument: select the blank coupling mode for channel 1, select the Target coupling mode for channel 2, and set the target to the designed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com