Multi-level quality control substance production method and traceability calibration method of blood gas analyzer
A technology for a blood gas analyzer and a calibration method, which is applied to the production method of multi-level quality control substances and the traceability calibration field of the blood gas analyzer, can solve the problems affecting the machine inspection qualification judgment, the inability to form an open standard, and the incomplete gas-liquid balance, etc. To achieve the effect of achieving accuracy and validity, realizing data comparison and judgment, and ensuring validity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]Step 1: Taking the preparation of 100ml fluorocarbon emulsion as an example, choose perfluorotributylamine 20%-40%w / v, perfluorotripropylamine 20%-40%w / v, perfluorodecalin 15%-30%w One of / v is used as a matrix substance, where w / v is a commonly used unit, representing mass concentration, that is, the mass per unit volume; the configuration methods of the three matrix substances are as follows:
[0053] (1), 30g of perfluorotributylamine; 1.5g of nonionic polyethylene glycol octyl phenyl ether; 0.1g of nonionic perfluorooctyl polyether surfactant; 2g of glycerol; High-speed shearing for 20 minutes, after primary emulsification, ultrasonic emulsification with 750W power for 60 minutes, the particle size of the fine emulsified emulsion is 0.1μm-0.15μm, and filtered with a 0.22μm filter membrane for use;
[0054] (2) Perfluorotripropylamine 35g; Nonionic polyoxyethylene sorbitan monolaurate 2g; Nonionic perfluorooctyl polyether surfactant 0.1g; Glycerin 2g; 2 High-pressure...
Embodiment 2
[0060] Embodiment 2: This embodiment also provides a traceable calibration method for a blood gas analyzer, comprising the following steps:
[0061] Step 1: Set a variety of different levels of quality control solutions, and set the K in each level of quality control solution + 、Na + , Ca 2+ , Cl - , lactic acid, glucose concentration and oxygen partial pressure, carbon dioxide partial pressure, H + , the concentration value of hematocrit, and configured through the steps in Example 1;
[0062] During the configuration process, each set level of quality control liquid corresponds to the configuration of steel gas cylinders with different gas components;
[0063] Step 2: Use a qualified blood gas analyzer to test the data of multiple quality control solutions at different levels, and compare and judge the detection data of each level of quality control solution with the corresponding set value; If the configuration is wrong, it needs to be reconfigured; if it is consistent...
Embodiment 3
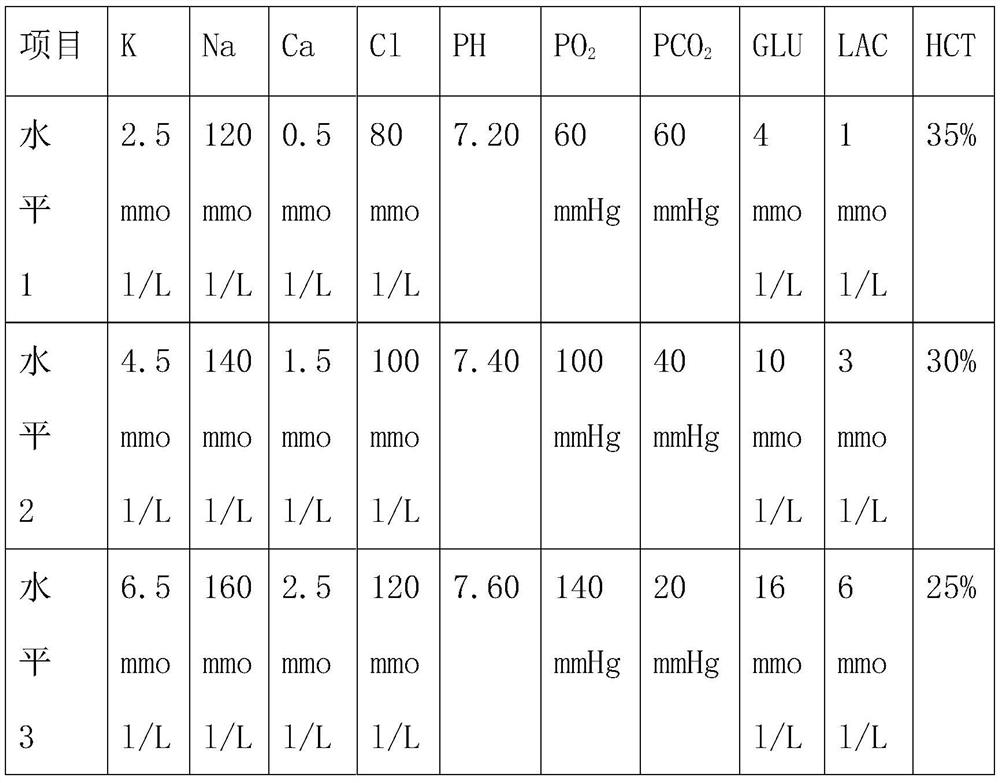
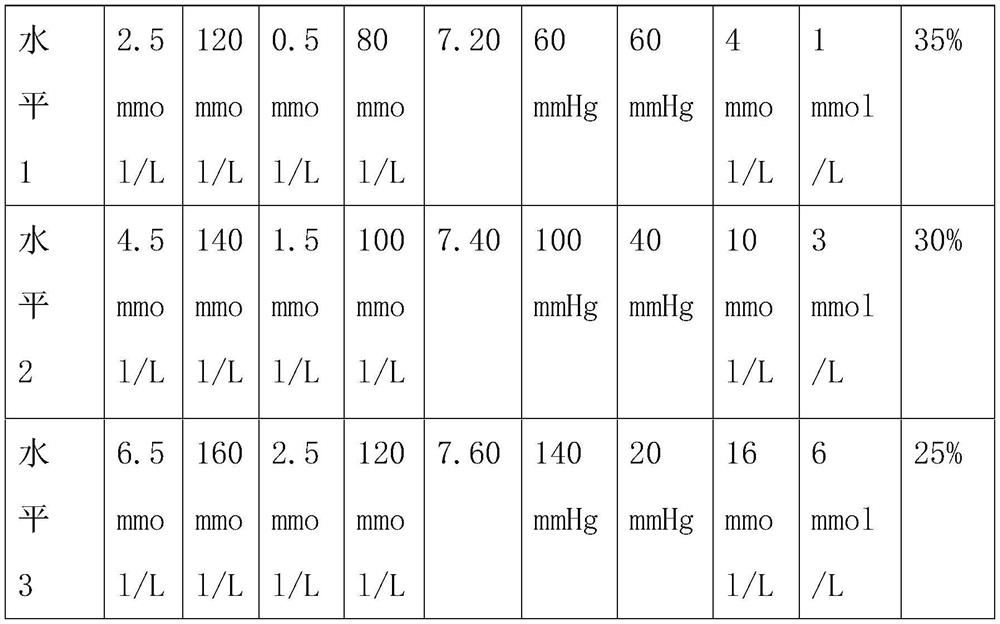
[0078] Embodiment 3: In order to verify the accuracy of the traceability calibration method of the present invention, three different levels of quality control substances are specially set, as shown in the following table:
[0079]
[0080] The steps to verify the accuracy and validity of the traceable calibration method are as follows:
[0081] Step 1: Through the formula in Example 2, PO in the quality control substance according to the above three levels of settings 2 , PCO 2 The numerical derivation of the gas combination ratio of the required corresponding configuration of the steel gas cylinder is shown in the table below:
[0082] PO 2
PCO 2
N 2
Level 1 Steel Gas Cylinders 8.4% 8.4% remaining Level 2 Steel Gas Cylinders 14.0% 5.6% remaining Level 3 Steel Gas Cylinders 19.6% 2.8% remaining
[0083] Steel gas cylinder manufacturers produce qualified steel gas cylinders according to the content of the above gas c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com