Fusion protein 3AN for capturing PRRSV nucleocapsid protein antibody and application thereof
A technology of nucleocapsid protein and fusion protein, which is applied in the direction of immunoglobulin, antiviral immunoglobulin, positive-sense single-stranded RNA virus, etc., and can solve the problems of detection of N protein antibody obstruction and lack of N protein antibody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038]In the present invention, the preparation method of the monoclonal antibody C8 preferably comprises the following steps: combining the coding sequences of the heavy chain variable region and the light chain variable region of the monoclonal antibody C8 with the pig IgG heavy chain and light chain respectively The constant region sequences of the chains were connected and inserted into the pcDNA3.4 eukaryotic expression vector respectively, and the heavy chain recombinant expression plasmid and the light chain recombinant expression plasmid were mixed and transfected into CHO suspension culture cells at a molar ratio of 2:3, and the complete antibody was obtained by expression. Purified by affinity chromatography. The present invention does not specifically limit the recombinant expression plasmid transfection method, expression method and affinity chromatography purification method, and the technical solutions well known in the art can be adopted.
[0039] In the present...
Embodiment 1
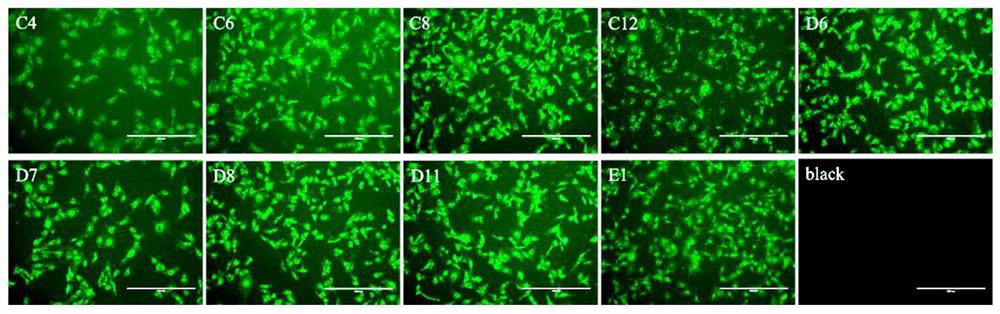
[0063] Preparation and Identification of Single B Cell Antibody to PRRSVN Protein from Porcine
[0064] Pigs were immunized with the commercial attenuated PRRS vaccine and the laboratory-made vaccine against the prevailing strain. At the same time, the whole virus antigen was purified, and the virus particles were labeled with biotin; after the eighth immunization, the venous blood of pig 0922# was collected, and the mononuclear cells (PBMCs) in the peripheral blood were separated, and the biotin-labeled PRRSV was used as the capture antigen, and passed Antigen-specific antibody-secreting single B cells were sorted from PBMCs by flow cytometry. Through single B cell antibody gene amplification technology, the heavy chain and light chain variable region (VH and VL) gene sequences of pig IgG antibodies were obtained, and then the variable region gene sequences were inserted into the pcDNA3.4 true region containing the constant region of pig IgG antibodies. In nuclear vectors, c...
Embodiment 2
[0068] Expression and purification of PRRSV nucleocapsid protein fused with foot-and-mouth disease virus 3A protein epitope
[0069]The coding sequence of 3AN protein was cloned into the pET-28a(+) vector by gene synthesis, transformed into BL21 competent cells, and expanded to 500ml after picking a single clone, adding IPTG to a final concentration of 1mM, and inducing expression for 5h. Collect the bacteria by centrifugation, add 50ml ice-bathed IB washing solution (EDTA10mmoXXl / L, Tris-HCl 20mmol / L, pH 7.5, TritonX-1001%) to resuspend the precipitate, sonicate until the bacteria are completely lysed, and collect the supernatant by centrifugation. Add 50% High Affinity Ni-Charged ResinFF at a ratio of 4:1, and incubate at 200 r / min at 4°C overnight to allow complete specific binding of the target protein to Ni-NTA His Bind. Wash with pH 8.0 (NaH 2 PO 4 50mm / L, NaCl 300mM, Urea 8M, imidazole 10mM) wash the column 3 times, each time 2 times the volume, and then use 0.5 time...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coating concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com