Method for synthesizing pentaerythritol tetra (3-lauryl thiopropionate) by one-pot method
A technology of laurel thiopropionate and pentaerythritol tetra, which is applied in the field of one-pot synthesis of pentaerythritol tetra, can solve the problems of a large amount of waste concentrated brine, difficulty in industrialization, low yield, etc., and achieve fast and efficient reaction and high-quality products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
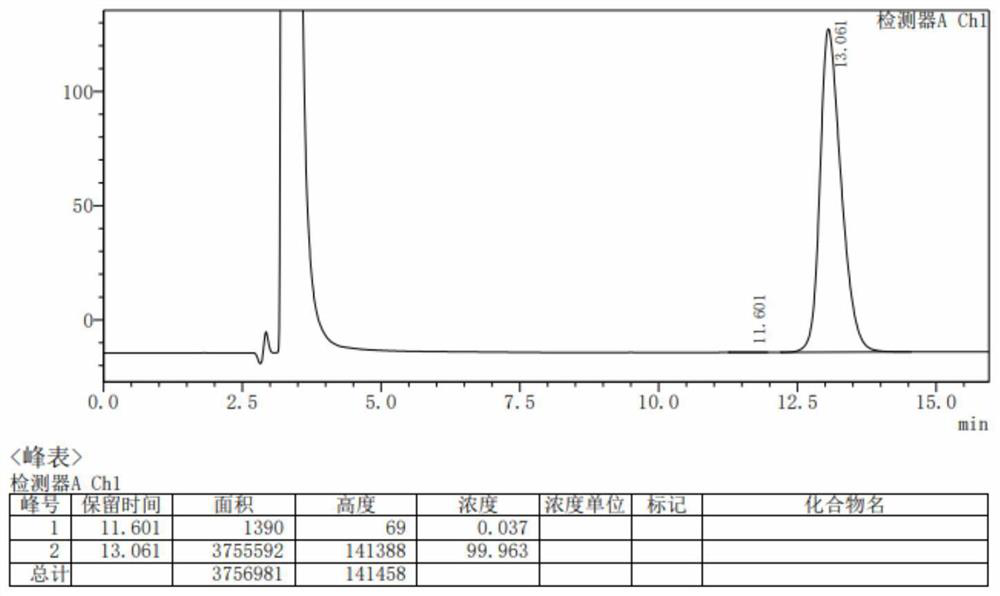
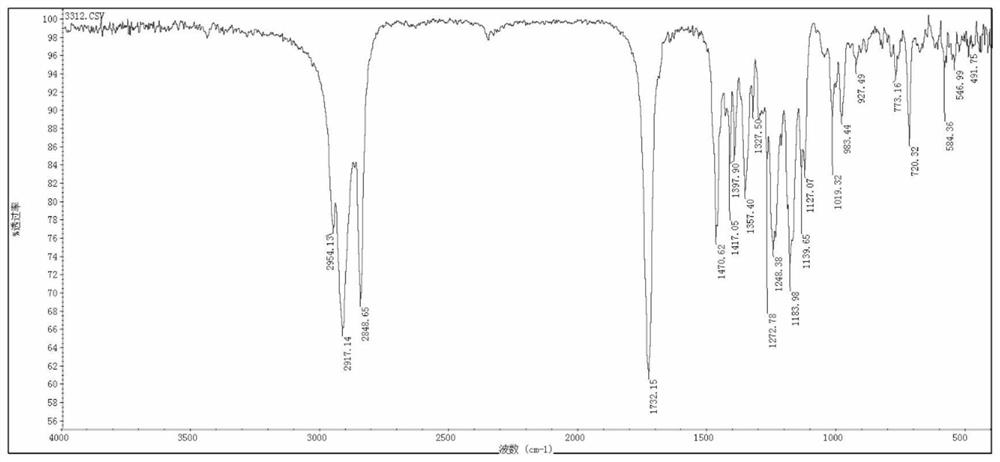
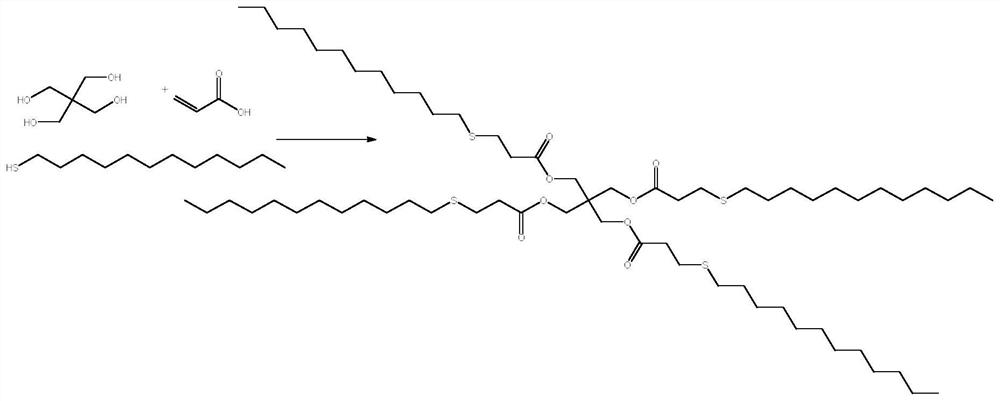
[0026] This example relates to a method for the one-pot synthesis of pentaerythritol tetrakis (3-lauryl thiopropionate). image 3 Shown: Include the following steps:
[0027] Step 1: Add reaction solvent to the reaction vessel, then add pentaerythritol, acrylic acid, triethylamine, and strong base catalyst in sequence, heat to 55°C, slowly add dodecanethiol dropwise, keep warm for 2 hours, and slowly add dropwise the reaction solution containing triphosgene Solution, insulated and stirred for 2h after the dropwise addition;
[0028] Step 2: Wash with water after the reaction, remove the solvent, add a crystallization solvent 10 times the mass of pentaerythritol, preferably one or more of methanol, ethanol, and isopropanol, and separate to obtain the product.
[0029] Specific steps are as follows:
[0030] Add 500kg of toluene to the 3000L reactor, add 100kg of pentaerythritol, 213.96kg of acrylic acid, 312.33kg of triethylamine, add 0.2kg of potassium methylate, slowly add ...
Embodiment 2
[0032] This example relates to a method for the one-pot synthesis of pentaerythritol tetrakis (3-lauryl thiopropionate). image 3 Shown: Include the following steps:
[0033] Step 1: Add reaction solvent to the reaction vessel, then add pentaerythritol, acrylic acid, triethylamine, and strong base catalyst in sequence, heat to 55°C, slowly add dodecanethiol dropwise, keep warm for 2 hours, and slowly add dropwise the reaction solution containing triphosgene Solution, insulated and stirred for 2h after the dropwise addition;
[0034] Step 2: After the reaction, wash with water, remove the solvent, add a crystallization solvent, and add a crystallization solvent 10 times the mass of pentaerythritol, preferably one or more of methanol, ethanol, and isopropanol to separate and obtain the product.
[0035] The specific steps are as follows: add 500kg of xylene to the 3000L reactor, add 100kg of pentaerythritol, 213.96kg of acrylic acid, 312.33kg of triethylamine, add 0.2kg of sodi...
Embodiment 3
[0037] This example relates to a method for the one-pot synthesis of pentaerythritol tetrakis (3-lauryl thiopropionate). image 3 Shown: Include the following steps:
[0038] Step 1: Add reaction solvent to the reaction vessel, then add pentaerythritol, acrylic acid, triethylamine, and strong base catalyst in sequence, heat to 55°C, slowly add dodecanethiol dropwise, keep warm for 2 hours, and slowly add dropwise the reaction solution containing triphosgene Solution, insulated and stirred for 2h after the dropwise addition;
[0039] Step 2: After the reaction, wash with water, remove the solvent, add a crystallization solvent, and add a crystallization solvent 10 times the mass of pentaerythritol, preferably one or more of methanol, ethanol, and isopropanol to separate and obtain the product.
[0040] The specific steps are as follows: add 500kg of methylcyclohexane to a 3000L reactor, add 100kg of pentaerythritol, 213.96kg of acrylic acid, 312.33kg of triethylamine, add 0.2k...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap