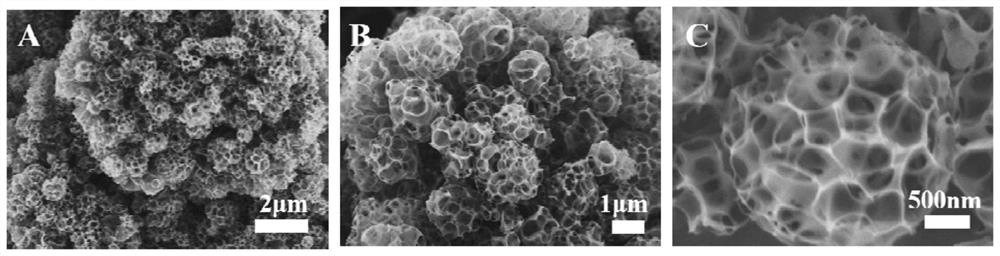
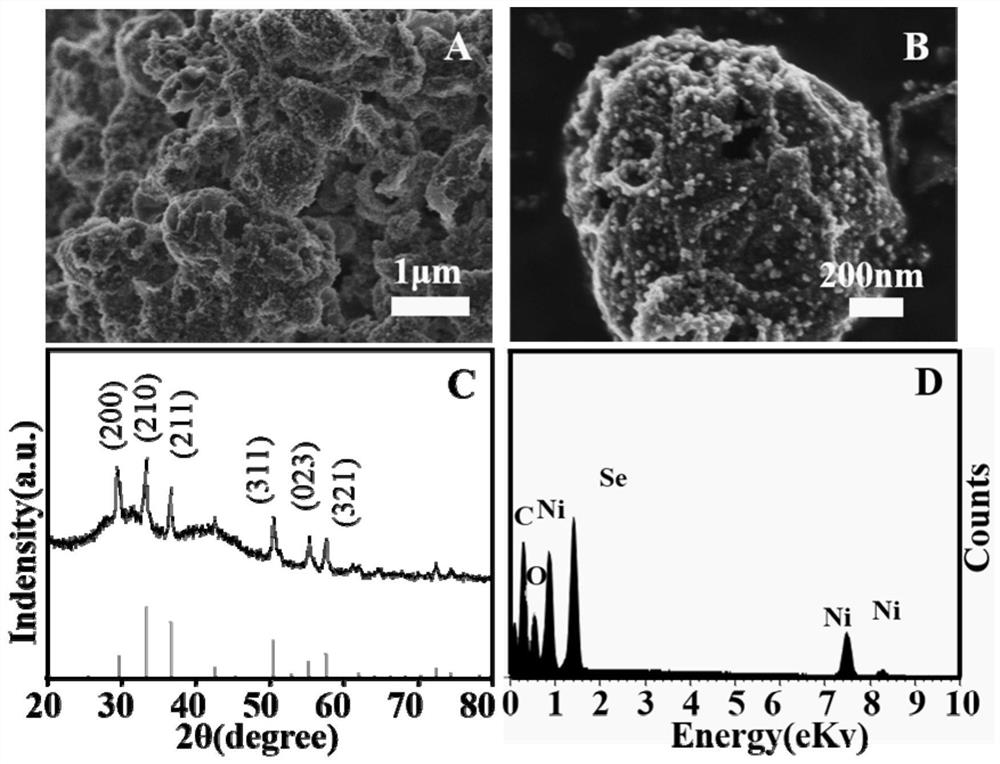
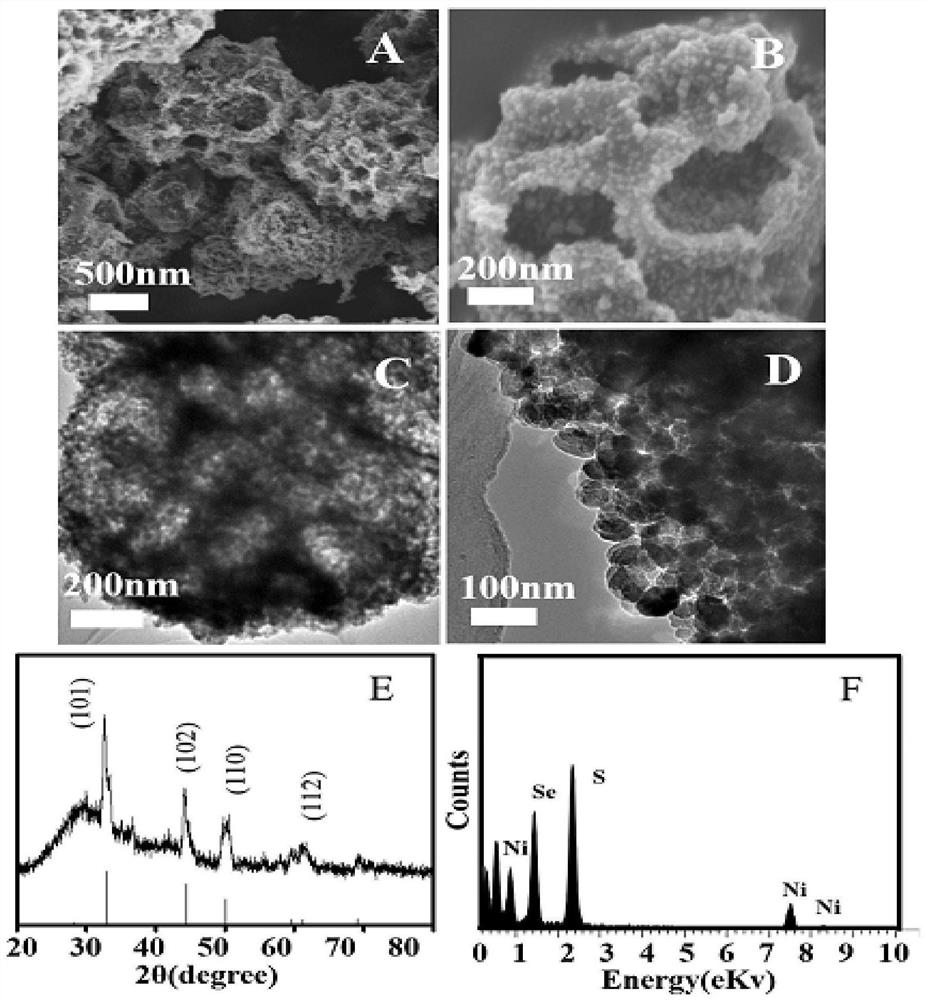
Preparation method and application of novel hydrogen evolution reaction catalyst Ni2SeS alloy nanorod modified porous carbon sphere composite material
A technology of hydrogen evolution reaction and composite materials, applied in the direction of physical/chemical process catalysts, chemical/physical processes, chemical instruments and methods, etc., can solve the problems of limited reserves and high cost of Pt-based noble metal catalysts, and achieve low industrial cost and abundant reserves , The effect of stable product shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] (1)NiSe 2 Preparation of / PCS
[0069] In the first step, 120 mg of nickel nitrate hexahydrate was weighed and dissolved in 30 mL of deionized water, and stirred at room temperature for 5 min. Pipette 20ml with a pipette gun and transfer to a polytetrafluoroethylene reaction kettle;
[0070] The second step is to accurately weigh 20.0 mg of porous carbon spheres and add them to the above reaction kettle, and disperse them uniformly by ultrasonic;
[0071] In the third step, add 43.58mg of Se powder to the above reaction kettle at room temperature, then inject 2ml of 80% hydrazine hydrate solution, stir for 30min to make it evenly mixed, and observe that the solution changes from green to purple suspension;
[0072] The fourth step is to place the reaction kettle in an electric constant temperature blast drying oven, raise the temperature from room temperature to 180°C at a rate of 2°C / min and keep it warm for 24 hours;
[0073] The fifth step is to wait until the rea...
Embodiment 2
[0085] Modulated NiSe 2 The ratio of nickel salt and carbon spheres in the preparation of / PCS.
[0086] (1)NiSe 2 Preparation of / PCS
[0087] In the first step, 120 mg of nickel nitrate hexahydrate was weighed and dissolved in 30 mL of deionized water, and stirred at room temperature for 5 min. Pipette 20ml with a pipette gun and transfer to a polytetrafluoroethylene reaction kettle;
[0088] The second step is to accurately weigh 80.0mg, 40.0mg, and 20.0mg of porous carbon spheres and add them to the above reaction kettle, and ultrasonically disperse them evenly;
[0089] In the third step, add 43.58mg of Se powder to the above reaction kettle at room temperature, then inject 2ml of 80% hydrazine hydrate solution, stir for 30min to make it evenly mixed, and observe that the solution changes from green to purple suspension;
[0090] The fourth step is to place the reaction kettle in an electric constant temperature blast drying oven, raise the temperature from room tempe...
Embodiment 3
[0099] Adjust Ni 2 NiSe in the preparation of SeS / PCS 2 / The ratio of PCS and sulfur powder.
[0100] (1)NiSe 2 Preparation of / PCS
[0101] In the first step, 120 mg of nickel nitrate hexahydrate was weighed and dissolved in 30 mL of deionized water, and stirred at room temperature for 5 min. Pipette 20ml with a pipette gun and transfer to a polytetrafluoroethylene reaction kettle;
[0102] The second step is to accurately weigh 20.0 mg of porous carbon spheres and add them to the above reaction kettle, and disperse them uniformly by ultrasonic;
[0103] In the third step, add 43.58mg of Se powder to the above reaction kettle at room temperature, then inject 2ml of 80% hydrazine hydrate solution, stir for 30min to make it evenly mixed, and observe that the solution changes from green to purple suspension;
[0104] The fourth step is to place the reaction kettle in an electric constant temperature blast drying oven, raise the temperature from room temperature to 180°C at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com