A coded microsphere containing cyanine compounds and its preparation method and application
A technology for encoding microspheres and compounds, which is applied in the field of new applications of cyanine compounds, can solve the problems of the limited number of fluorescent signal uniformity coding, the difficulty of selecting fluorescent dyes, and the unpredictable coding signal, etc., and achieves a large and uniform coding range Good performance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
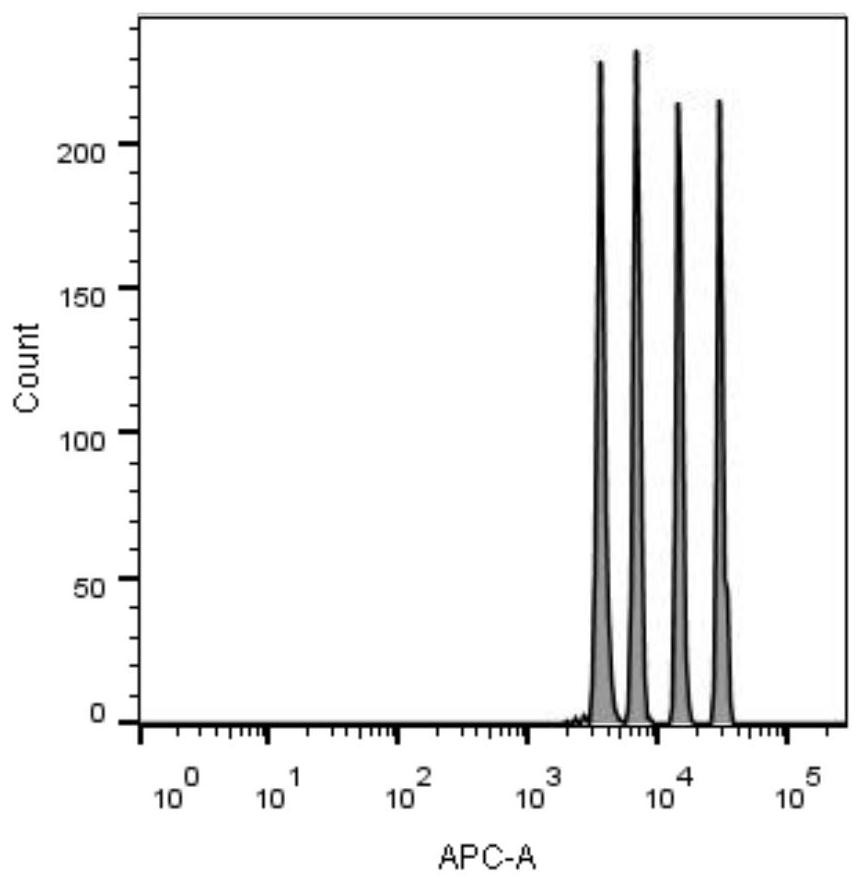
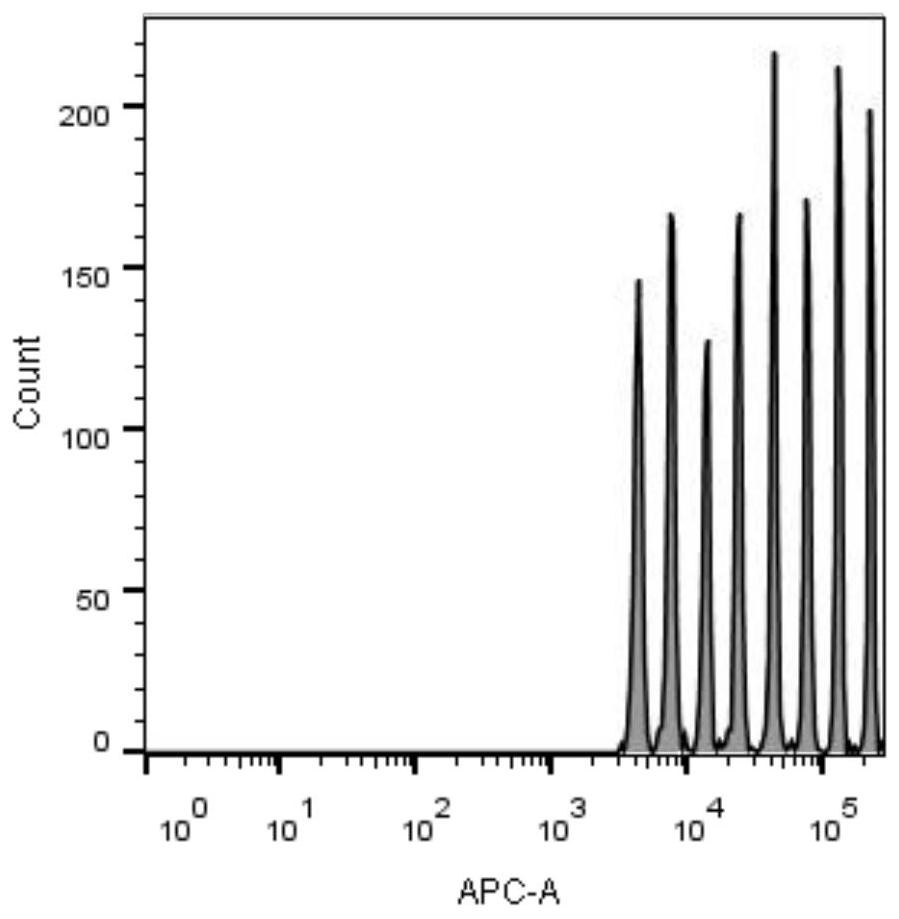
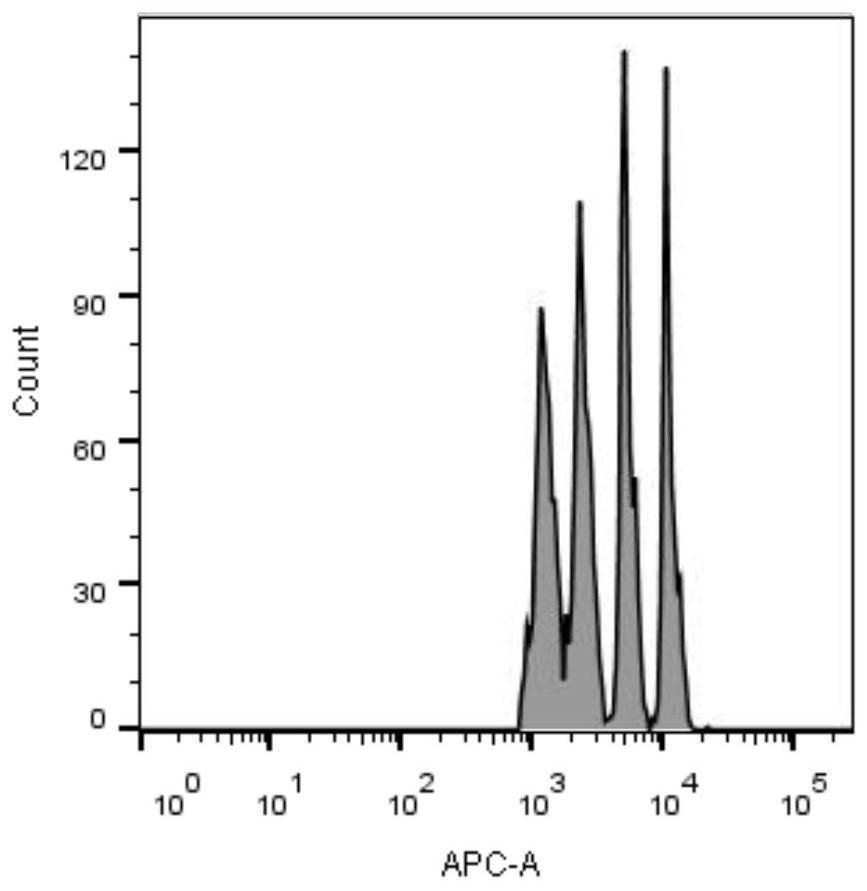
Image
Examples
preparation example 1
[0119] Preparation example 1 (preparation of compound I):
[0120]
[0121] 40mmol 2,3,3-trimethylindole, 80mmol propenyl iodide and 20mL xylene, heated to reflux under nitrogen protection, reacted for 24 hours, cooled to room temperature, added 200mL ether, filtered, and washed with appropriate amount of ether , to obtain a solid intermediate product;
[0122] Take 6 mmol of the solid intermediate product, 2 mmol of squaraine, 8 mL of toluene, 6 mL of n-butanol, and 6 mL of pyridine, stir and heat to reflux under the protection of argon, and stop the reaction after 6 hours. After cooling to room temperature, appropriate amount of solvent was distilled off under reduced pressure. 200 mL of diethyl ether was added thereto to precipitate the product, which was filtered, washed with diethyl ether and dried to obtain an unpurified solid product.
[0123] The unpurified solid product was purified by silica gel column chromatography, and was eluted with a gradient of eluent eth...
preparation example 2
[0124] Preparation example 2 (preparation of compound II):
[0125]
[0126] 40mmol 1,1,2-trimethyl-1H-benzo[e]indole, 80mmol 1-bromopropane and 25mL o-dichlorobenzene were heated to reflux under the protection of argon, reacted for 40 hours, cooled to room temperature, 200 mL of ethyl acetate was added thereto, and the product was precipitated by ultrasonic oscillation, filtered, and washed to obtain a solid intermediate product;
[0127] Take 6 mmol of the solid intermediate product, 2 mmol of squaraine, 8 mL of toluene, 6 mL of n-butanol, and 6 mL of pyridine, stir and heat to reflux under the protection of argon, and stop the reaction after 6 hours. After cooling to room temperature, part of the solvent was distilled off under reduced pressure. 200 mL of diethyl ether was added thereto to precipitate the product, which was filtered, washed with diethyl ether and dried to obtain an unpurified solid product.
[0128] The unpurified solid product was purified by silica g...
preparation example 3
[0130] Preparation example 3 (preparation of compound III):
[0131]
[0132] 40mmol 2,3,3-trimethylindole, 80mmol 6-bromohexanoic acid ethyl ester and 25mL o-dichlorobenzene were heated to reflux under argon protection, reacted for 24 hours, cooled to room temperature, and 150mL acetic acid was added to it Ethyl ester, ultrasonic vibration to precipitate the product, grind in ethyl acetate and filter to obtain a dark brown-red block intermediate product;
[0133] Take 6 mmol of the brown-red intermediate product, 2 mmol of squaraine, 8 mL of benzene, 6 mL of n-butanol, and 6 mL of pyridine, stir and heat to reflux under the protection of argon, and stop the reaction after 6 hours. After cooling to room temperature, 200 mL of diethyl ether was added to precipitate the product, filtered, washed with diethyl ether and dried to obtain a dark blue solid.
[0134] The dark blue solid was purified by silica gel column chromatography, and the eluent was ethyl acetate:petroleum et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com