Application of sophoridine in preparation of anti-herpes virus medicine
A technology of herpes virus and herpes simplex virus, which is applied in the direction of antiviral agents, drug combinations, diseases, etc., and can solve problems such as complex components, unstable active ingredient content and activity intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Plaque test to detect the inhibitory effect of drugs on viral plaques
[0038] 1. Experimental method
[0039] Determination of virus titer: Vero cells in the logarithmic growth phase were taken, digested with trypsin and centrifuged, counted, and counted according to 1.3×10 5 Seed / ml density to 96-well plate, 100 μl per well, place at 37°C, 5% CO 2 cultured in an incubator. Discard the supernatant after the cells grow into a monolayer, and dilute the virus stock solution to 10 times with the maintenance solution. -1 to 10 -9 For a series of gradients, 6 replicate wells were set for each concentration, and 100 μl of virus dilution per well was inoculated on Vero cells, at 37°C, 5% CO 2 Adsorb in the incubator for 2 hours, discard the supernatant, add 100 μl of maintenance solution to each well to continue the culture, and set a normal cell control group. The cytopathic effect (CPE) was observed and recorded under an inverted microscope every day, and the...
Embodiment 2
[0043] Example 2: Plaque test method to detect the influence of drugs on the virulence of progeny viruses
[0044] 1. Experimental method
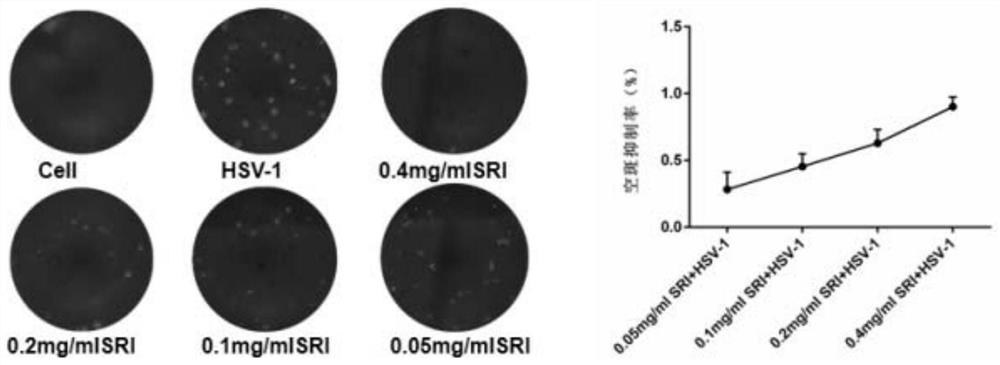
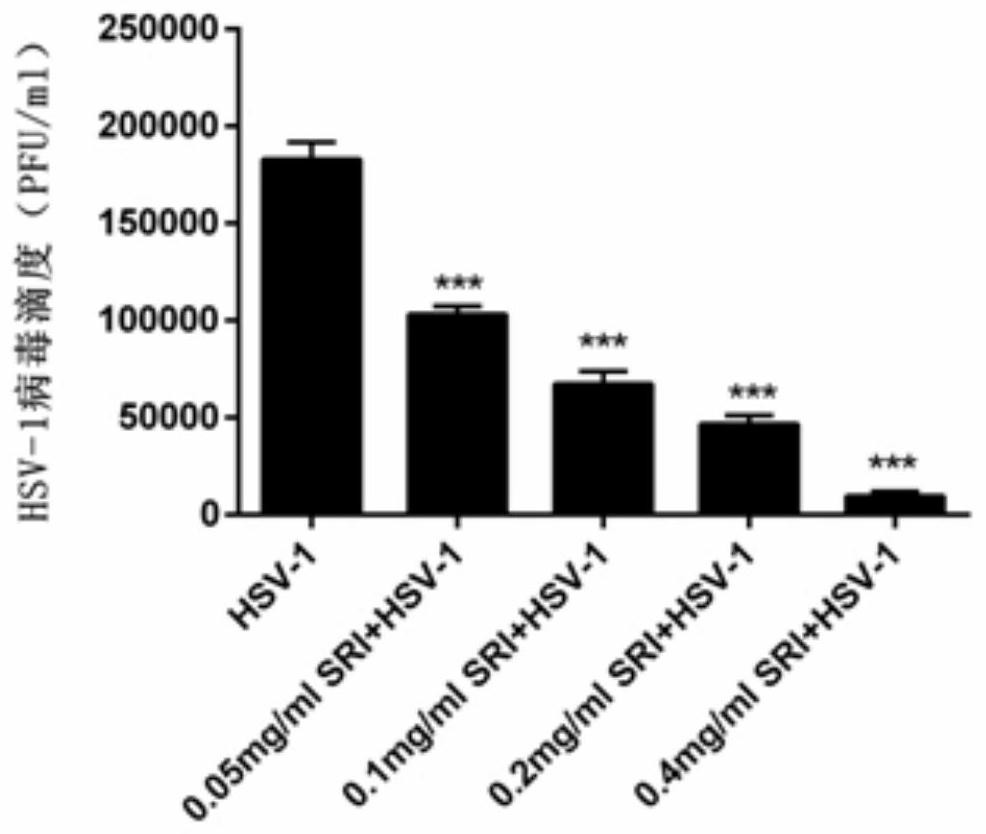
[0045] Seed Vero cells on a 12-well plate at an appropriate density. After the cells are fused the next day, add HSV-1 to infect the cells. Place them in a 5% CO2 culture at 37°C for 2 hours. Discard the supernatant and add different concentrations of The cells were treated with sophoridine and placed in the incubator for 24 hours. After culturing for 24 hours, discard the supernatant and add 1ml of maintenance solution to each well, transfer the 12-well plate to -80°C and 37°C for three times of freezing and thawing to release virus particles, collect the supernatant and place in Standby at -80°C. The virus titer of progeny was determined by plaque test.
[0046] 2. Experimental results
[0047] Plaque test results such as image 3 As shown, it can be seen that sophoridine has a significant inhibitory effect on the generation of HSV-...
Embodiment 3
[0048] Embodiment 3: Western blotting test detects the impact of drugs on viral protein gB, gD expression
[0049] 1. Experimental method
[0050] Inoculate vero cells or Hela cells in 6-well plates, grow into a single layer, and use 100TCID 50 Infect the cells with HSV-1 virus liquid, absorb in a 37°C, 5% CO2 incubator for 2 hours, discard the supernatant, add gradient diluted sophoridine solutions of different concentrations after washing with PBS, and culture in a 37°C, 5% CO2 incubator 24h. After 24 hours, the supernatant was discarded, and pre-cooled PBS was added to wash twice, each time for 3 minutes. Add 120 μL of RIPA lysate (containing 1% protease inhibitor and 1% phosphatase inhibitor), and lyse on ice for 30 min. During this period, gently pipette with a pipette to make the lysate fully contact with the cells. After fully lysed, centrifuge at 12000g, 4°C for 15min. The supernatant was taken, and the total protein content was determined using the BCA protein qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com