A kind of indanone imine derivative and its preparation method and application
A technology of derivatives and imines, which is applied in the field of indenone imine derivatives and their preparation, can solve the problems of lack of effective treatment of tumors and anti-tumor drugs that cannot meet the treatment requirements, and achieve good anti-tumor effects and high atomic economy The effect of novelty and structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of indanone imine derivatives
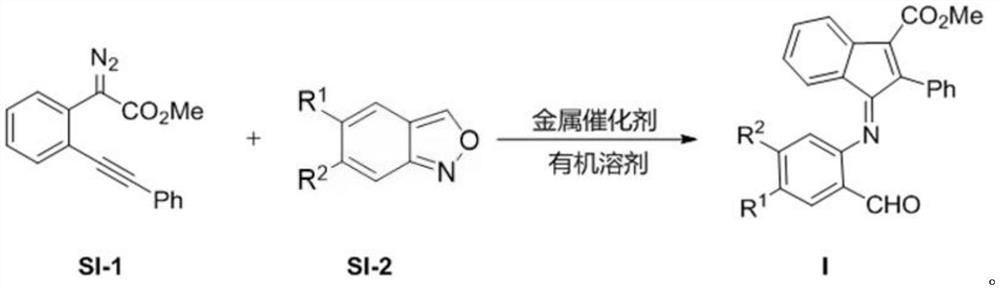
[0039] The preparation method of indanone imine derivative is carried out according to the following reaction formula:
[0040]
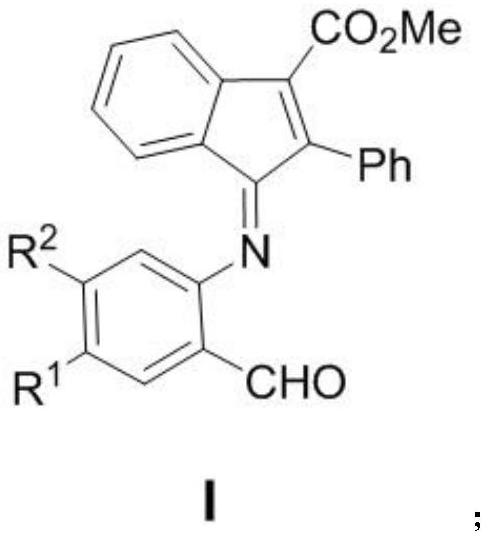
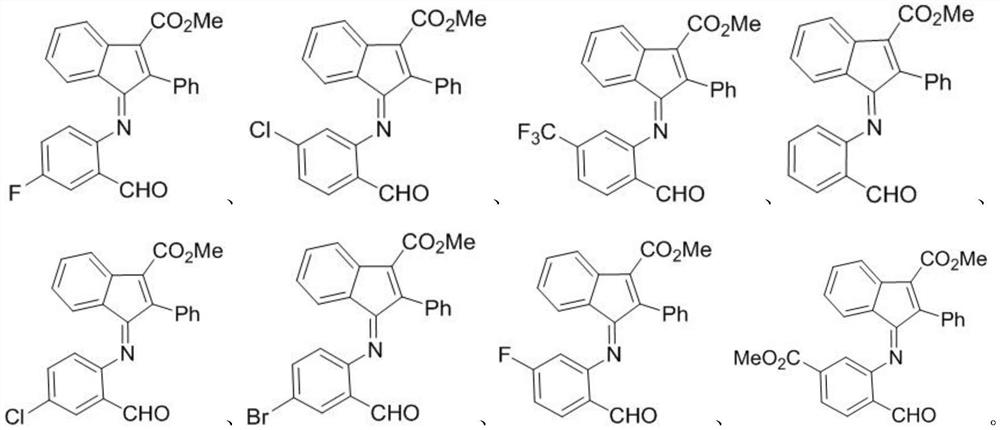
[0041] where the R 1 For hydrogen, fluorine, chlorine, bromine; R 2 For hydrogen, fluorine, chlorine, trifluoromethyl, methoxycarbonyl.
[0042] The specific preparation method of the indanone imine derivative is as follows: the diazo compound (0.20 mmol) represented by the formula SI-1 in the above reaction formula, the benzisoxazole (0.24 mmol) represented by the formula SI-2 and a catalyst [2-(Dicyclohexylphosphine)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-diphenyl]bis(trifluoromethanesulfonylidene) Amine) gold (0.01 mmol) was weighed in a test tube, then 2 mL of anhydrous 1,2-dichloroethane was added to the reaction system, and the reaction was stirred at 60 ° C for 24 hours until the diazo compound was completely consumed; Filtration, column chromatography separation and purifi...
Embodiment 2
[0055] Example 2 Inhibitory activity of indanone imine derivatives on colorectal adenocarcinoma cells
[0056] 1. The human colorectal adenocarcinoma cells used in the assay are colorectal adenocarcinoma cells (HCT-116) (purchased from Guangzhou Saiku Biotechnology Co., Ltd.).
[0057] 2. The CCK-8 method was used to determine the inhibitory effect of indanone imine derivatives on the proliferation of human colorectal adenocarcinoma cells, wherein the specific determination process of the inhibition rate of HCT-116 cells was as follows:
[0058] 1) Add 100 μL of cell suspension prepared with complete medium to the 96-well plate (the cell inoculation amount is 5000 cells / well), and add 100 μL of cell culture medium without cells to the blank well as a control. The 96-well culture plate was pre-incubated in an incubator (37°C, 5% CO) for 24 hours. 2 );
[0059] 2) Add 1.0 μL solution of the compounds to be tested (compounds I-1 to I-8) dissolved in DMSO to the culture plate to...
Embodiment 3
[0074] Example 3 Inhibitory activity of indanone imine derivatives on osteosarcoma cells
[0075] 1. The human osteosarcoma cells used in the assay are: human osteosarcoma cells (SJSA-1) (purchased from Guangzhou Saiku Biotechnology Co., Ltd.).
[0076] 2. The CCK-8 method was used to determine the inhibitory effect of indanone imine derivatives on the proliferation of human osteosarcoma cells, wherein the specific determination process of the inhibition rate of SJSA-1 cells was as follows:
[0077] 1) Add 100 μL of cell suspension prepared with complete medium to the 96-well plate (the inoculation amount of cells is 5000 cells / well), and add 100 μL of cell culture medium without cells to the blank well as a control. The 96-well culture plate was pre-incubated in an incubator (37°C, 5% CO) for 24 hours. 2 );
[0078] 2) Add 1.0 μL solution of the compounds to be tested (compounds 1-1 to 8) dissolved in DMSO to the culture plate to make the final concentration 20 μM, and add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com