Steroidal intermediate chemical as well as preparation method and application thereof
A chemical and steroid technology, applied in the field of steroid intermediate chemicals and their preparation, can solve the problems of limited industrial and social benefits, unreasonable selection of starting materials, and difficult recovery of solvents, and achieves low raw material prices, The effect of shortening the synthesis route, stable synthesis process and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
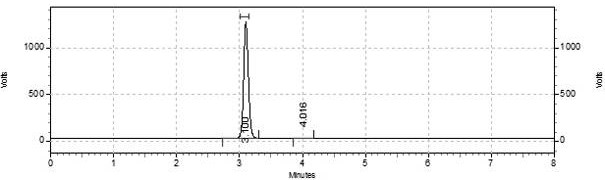
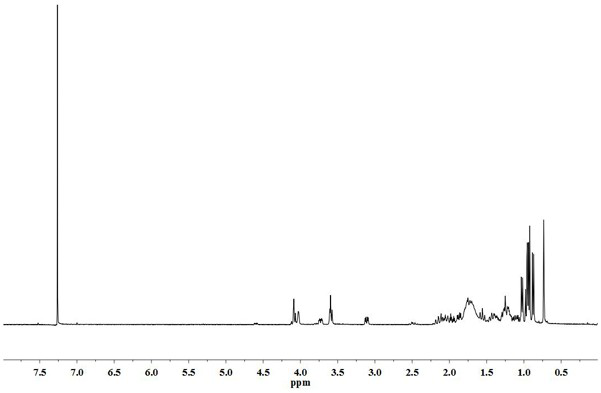
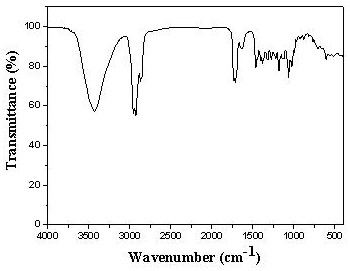
[0027] Dissolve 4g stigmasterol (9.7mmol) and 1.19g triethylamine (11.7mmol) in 40mL butanone. After the dissolution is complete, slowly add 2.38g p-toluenesulfonyl chloride (12.5mmol) dropwise. After the dropwise addition, Continue to react at room temperature for 4 hours under stirring, monitor the progress of the reaction with a TLC plate, and confirm the reaction end point with HPLC when it is close to the end point. The product does not need to be separated and purified for the next step reaction. After the end, it is distilled under reduced pressure to recover butanone.
[0028] Dissolve the product of the previous step in 120mL butanone, then add 40mL KHCO 3 (10mmol) solution, the temperature is controlled at 60-80°C, and the end point of the reaction is confirmed by TLC plate and HPLC. After the end, butanone is distilled off, and 20mL of petroleum ether is added to wash until neutral, and the organic phase is combined to enter the next step of reaction.
[0029] Slowl...
Embodiment 2
[0038] The difference between embodiment 2 and embodiment 1 is: after the rearrangement reaction, slowly add 1.27gMnO under stirring in the system 2 (14.6mmol), the temperature was controlled at 60°C, and the reaction was carried out for 3h. 2.80 g (yield: 70.6%) of high-purity steroidal intermediates were obtained.
Embodiment 3
[0040] The difference between embodiment 3 and embodiment 1 is: after the rearrangement reaction, slowly add 0.84gMnO under stirring in the system 2 (9.7mmol), control the temperature at 60°C, and react for 3h. Obtain high-purity steroidal intermediate chemical 2.58g (yield is 64.8%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com