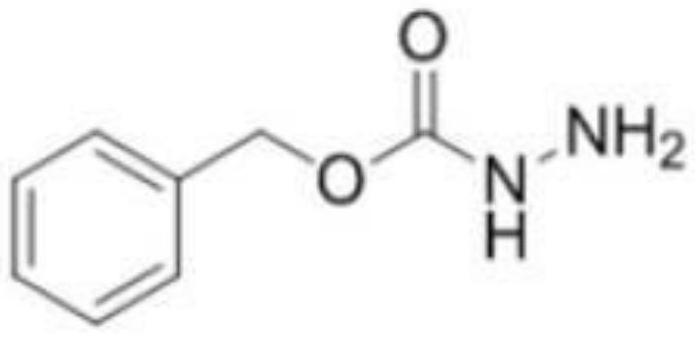
Method for preparing benzyl carbazate
A technology of benzyl carbazate and benzyl chloroformate, which is applied in the field of preparing benzyl carbazate, can solve the problems of low yield of benzyl carbazate, low yield of transesterification, difficult purification of products, etc., and achieve degradation Controllable, waste-free, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
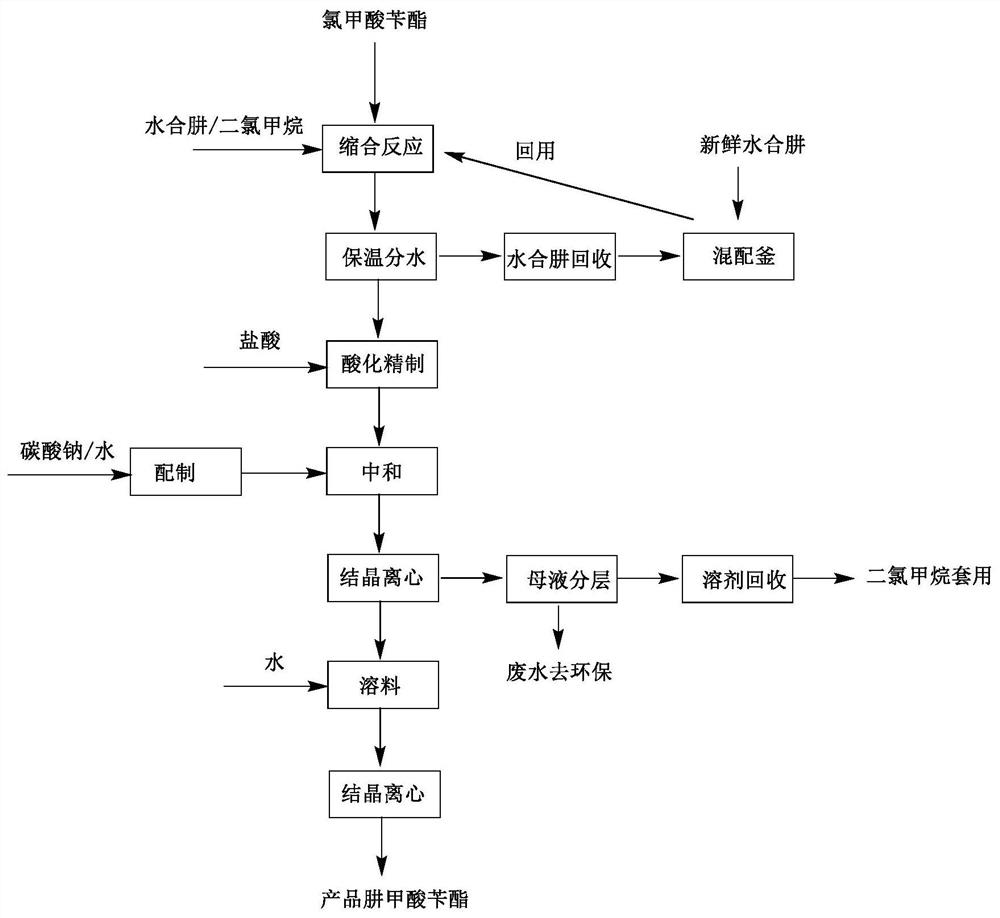
[0034] The technical process of the preparation method of benzyl carbazate in the present invention is as figure 1 shown.
[0035] A method for preparing benzyl carbazate, comprising the steps of:
[0036] The first step: add dichloromethane solvent into the reaction kettle, then add hydrazine hydrate and stir until dissolved, then add the catalyst, and continue to stir for at least 5 minutes; the hydrazine hydrate and solvent are in the order of 1:6 by weight; the catalyst is potassium carbonate , sodium carbonate, lithium carbonate, triethylamine, potassium hydroxide or sodium hydroxide;
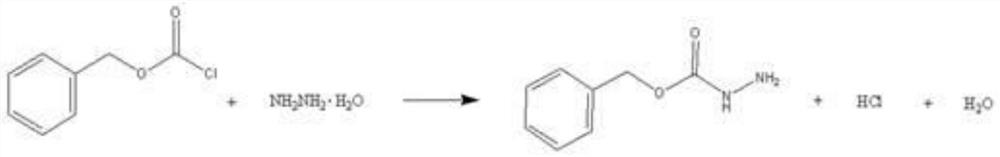
[0037] The second step: slowly drop benzyl chloroformate into the reaction kettle at 20±2°C, the dropping time is controlled at 10 minutes, and continue to keep warm for 2 hours; hydrazine hydrate, catalyst and benzyl chloroformate are in sequence according to the molar ratio 16:1:4;
[0038] The third step: heat-preserve and divide the obtained reaction liquid through a liquid separati...
Embodiment 2
[0046] A method for preparing benzyl carbazate, comprising the steps of:
[0047] The first step: add dichloromethane solvent into the reaction kettle, then add hydrazine hydrate and stir until dissolved, then add the catalyst, and continue stirring for at least 5 minutes; the hydrazine hydrate and solvent are in the order of 1:7 by weight; the catalyst is potassium carbonate , sodium carbonate, lithium carbonate, triethylamine, potassium hydroxide or sodium hydroxide;
[0048] Step 2: Slowly add benzyl chloroformate to the reaction kettle dropwise at 25±2°C, and the dropping time is controlled at 12 minutes, and continue to keep warm for 2 hours; The ratio is 16:1:4 in turn;
[0049] The third step: heat-preserve and divide the obtained reaction liquid through a liquid separation device, recover the hydrazine hydrate after the water division, and use the organic phase obtained after the water division for subsequent use;
[0050] Step 4: add hydrochloric acid with a concent...
Embodiment 3
[0057] A method for preparing benzyl carbazate, comprising the steps of:
[0058] The first step: add dichloromethane solvent into the reaction kettle, then add hydrazine hydrate and stir until dissolved, then add the catalyst, and continue to stir for at least 5 minutes; the hydrazine hydrate and solvent are in the order of 1:8 by weight; the catalyst is potassium carbonate , sodium carbonate, lithium carbonate, triethylamine, potassium hydroxide or sodium hydroxide;
[0059] The second step: slowly drop benzyl chloroformate into the reaction kettle at 20±2°C, the dropping time is controlled at 15 minutes, and continue to keep warm for 2 hours; The ratio is 16:1:4 in turn;
[0060] The third step: heat-preserve and divide the obtained reaction liquid through a liquid separation device, recover the hydrazine hydrate after the water division, and use the organic phase obtained after the water division for subsequent use;
[0061] Step 4: add hydrochloric acid with a concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com