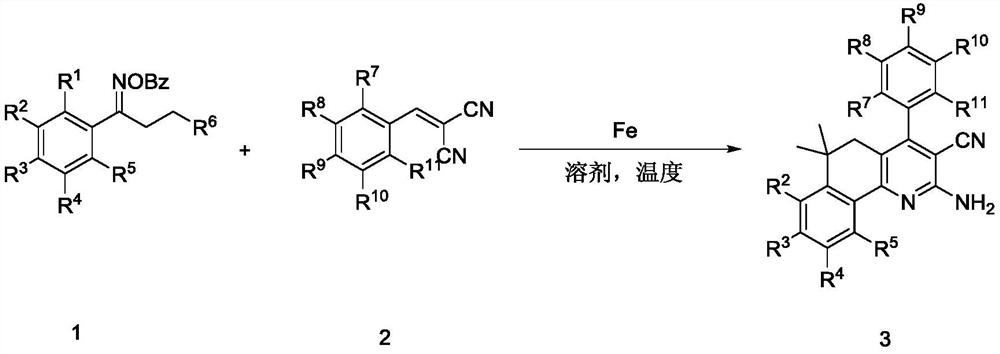
Preparation method of 2-amino fused pyridine compound
An amino-based fused pyridine and compound technology, which is applied in the field of preparation of 2-amino-based fused pyridine compounds, can solve the problems of difficulty in storage, poor stability of reaction substrates, narrow substrate selection range, etc., and achieves easy storage and performance. Superior, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
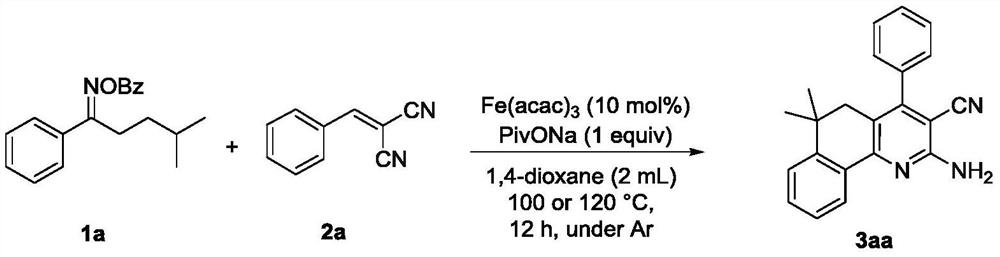
[0018] Take a 20mL reaction tube, add benzoxime ester compound 1a (0.6mmol), malononitrile-derived olefin compound 2a (0.2mmol), iron triacetylacetonate 0.01mmol, under the protection of inert gas (nitrogen or argon) , after addition of dioxane (2 mL), the reaction tube was sealed with a Teflon stopper. Place the reaction tube in a stirred oil bath at 110°C for 12 hours, and detect it by TLC. After the reaction is completed, cool down to room temperature, filter with diatomaceous earth, and then rinse with 20 mL of ethyl acetate for several times until the filtrate is colorless, and combine the organic phases , spin to remove the solvent to obtain a mixture containing 2-amino-6,6-dimethyl-4-phenyl-5,6-dihydrobenzo[h]quinoline-3-carbonitrile (3aa), and then flash column chromatography to obtain the product 3aa. Rate 90%.
[0019] 1 H NMR (600MHz, CDCl 3 )δ8.32(d,J=7.8Hz,1H),7.53-7.49(m,3H),7.43–7.41(m,1H),7.37–7.35(m,2H),7.31(d,J=6.9Hz ,2H),5.16(s,2H),2.53(s,2H)...
Embodiment 2
[0021]
[0022] Take a 20mL reaction tube, add benzoxime ester compound 1a (0.6mmol), malononitrile-derived olefin compound 2a (0.2mmol), ferrous acetylacetonate 0.01mmol, under the protection of inert gas (nitrogen or argon) , after addition of dioxane (2 mL), the reaction tube was sealed with a Teflon stopper. Place the reaction tube in a stirred oil bath at 110°C for 12 hours, and detect it by TLC. After the reaction is completed, cool down to room temperature, filter with diatomaceous earth, and then rinse with 20 mL of ethyl acetate for several times until the filtrate is colorless, and combine the organic phases , spin to remove the solvent to obtain a mixture containing 2-amino-6,6-dimethyl-4-phenyl-5,6-dihydrobenzo[h]quinoline-3-carbonitrile (3aa), and then flash column chromatography to obtain the product 3aa, harvested rate of 65%.
[0023] 1 H NMR (600MHz, CDCl 3 )δ8.32(d,J=7.8Hz,1H),7.53-7.49(m,3H),7.43–7.41(m,1H),7.37–7.35(m,2H),7.31(d,J=6.9Hz ,2H),5.16(s,2...
Embodiment 3
[0025]
[0026] Take a 20mL reaction tube, add benzoxime ester compound 1a (0.6mmol), malononitrile derivative olefin compound 2a (0.2mmol), ferric chloride 0.01mmol, under the protection of inert gas (nitrogen or argon), After addition of dioxane (2 mL), the reaction tube was sealed with a Teflon stopper. Place the reaction tube in a stirring oil bath at 110°C for 12 hours, and detect it by TLC. After the reaction is completed, cool down to room temperature, filter with diatomaceous earth, and then rinse with 20 mL of ethyl acetate for several times until the filtrate is colorless, and combine the organic phases , spin to remove the solvent to obtain a mixture containing 2-amino-6,6-dimethyl-4-phenyl-5,6-dihydrobenzo[h]quinoline-3-carbonitrile (3aa), and then flash column chromatography to obtain the product 3aa, harvested The rate is 79%.
[0027] 1 H NMR (600MHz, CDCl 3 )δ8.32(d,J=7.8Hz,1H),7.53-7.49(m,3H),7.43–7.41(m,1H),7.37–7.35(m,2H),7.31(d,J=6.9Hz ,2H),5.16(s,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com