Preparation method of brivaracetam intermediate
A technology of an intermediate and an operation method, which is applied in the field of preparation of the intermediate-3-propyl-γ-butyrolactone, can solve the problems of few reaction steps and poor stereoselectivity, and achieve high overall yield and reaction good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
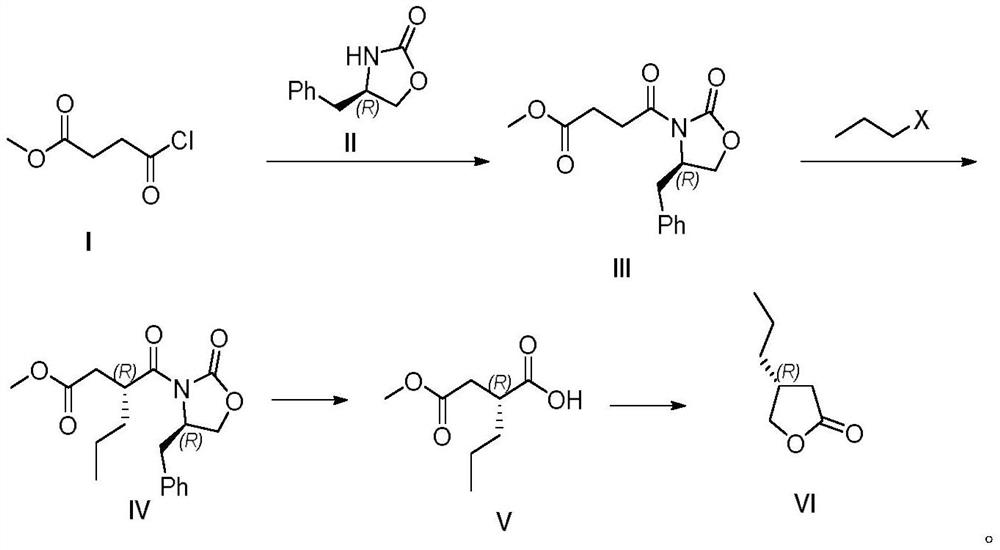
[0058] Embodiment 1: the preparation of compound III
[0059] Add compound II (0.88g, 5.00mmol) to a dry 50mL round bottom flask, add dichloromethane (10mL) and stir to dissolve, add triethylamine (0.76g, 7.50mmol) at room temperature, cool to 0°C in an ice bath , slowly added a dichloromethane solution of compound I (0.90 g, 6.00 mmol), reacted for 2 hours, and detected the reaction by TLC. After the reaction, dichloromethane (15mL) was added for dilution, washed with saturated sodium bicarbonate solution (15mL) and separated, the aqueous phase was extracted with dichloromethane (30mL×2), and the organic phase was successively washed with saturated brine. Dry over sodium sulfate, concentrate under reduced pressure, and purify by column chromatography (petroleum ether / ethyl acetate=10:1 to 2:1 gradient elution) to obtain a white solid (1.05 g), with a reaction yield of 72%.
[0060] White solid, m.p.90-91℃; R f =0.27(PE:EA=3:1); 1 H NMR (500MHz, CDCl 3 ):δ=7.21-7.36(m,5H),...
Embodiment 2
[0061] Embodiment 2: the preparation of compound III
[0062] Take a dry 50mL round bottom flask, add compound II (0.88g, 5.00mmol) and sodium hydride (60% dispersed in paraffin oil, 0.30g), add tetrahydrofuran (10mL) and stir to dissolve, cool to 0 ° C in an ice bath, Compound I (0.90 g, 6.00 mmol) was added, and the stirring reaction was continued at 0° C. for 2 h, and the reaction was detected by TLC. After the reaction was completed, the reaction was quenched with 1mol / L hydrochloric acid, extracted with dichloromethane (30mL×2), the organic phases were combined, and the mixed organic phases were successively washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. Purified by column chromatography (petroleum ether / ethyl acetate=10:1 to 2:1 gradient elution), a white solid compound (1.14 g) was obtained, and the reaction yield was 78%. The characterization data of the obtained white solid is consistent with that of Example...
Embodiment 3
[0063] Embodiment 3: the preparation of compound III
[0064] Add compound II (0.88g, 5.00mmol) to a dry 50mL round bottom flask, add dichloromethane (10mL) and stir to dissolve, add triethylamine (0.76g, 7.50mmol) and DMAP (0.31g, 0.25 mmol), the temperature was cooled to 0° C. in an ice bath, and a dichloromethane solution of Compound I (0.90 g, 6.00 mmol) was slowly injected, reacted for 2 hours, and the reaction was detected by TLC. After the reaction, dichloromethane (15mL) was added for dilution, washed with saturated sodium bicarbonate solution (15mL) and separated, the aqueous phase was extracted with dichloromethane (30mL×2), and the organic phase was successively washed with saturated brine. Dry over sodium sulfate, concentrate under reduced pressure, and purify by column chromatography (petroleum ether / ethyl acetate=10:1 to 2:1 gradient elution) to obtain a white solid (1.05g), with a reaction yield of 83%. The characterization data of the obtained white solid is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com