Application of chemical modification CRISPR/Cpf1 compound
A chemical modification and complex technology, applied in the field of gene editing, can solve the problems of off-target, low efficiency of CAR-T cell preparation, long expression peak, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] In the embodiments of the present invention, a gene editing method is provided. In this method, a chemically modified CRISPR / Cpf1 complex is first prepared. The preparation method of the complex comprises: (1) expressing Cpf1 protein containing unnatural amino acid by gene codon extension technology; (2) coupling Cpf1 protein containing modified crRNA and unnatural amino acid at 5' end through orthogonal reaction couplet. The complex is then used to edit the cell.
[0053] In the gene editing method of the present invention, the gene editing method mediated by adeno-associated virus vector, the gene editing method mediated by lentivirus vector, the gene editing method mediated by adenovirus are also combined, or a plasmid vector or a single-stranded / Double-stranded DNA is introduced into the target gene. Alternatively, RNA interference technology can also be combined to reduce or silence the expression of the target gene. For example, in the embodiment of the prese...
Embodiment 1
[0066] Example 1: Construction of a gene vector comprising site-directed mutation AsCpf1
[0067] (1) Acquisition of helper plasmids
[0068] pUltra-MjPolyRS (purchased from Addgene) (hereinafter referred to as the helper plasmid), which can express tRNA and tRNA synthetase for specifically recognizing and inserting the unnatural amino acid AeF.
[0069] (2) Acquisition of AsCpf1 plasmid
[0070] The protein sequence in the pET22b-AsCpf1 plasmid was obtained by PCR from Addgene plasmid #102565, and then PCR primers were used to introduce enzyme-cut 5'NdeI and 3'XhoI at the end, and the complete plasmid sequence was constructed by enzyme-cut ligation, which can be used in Escherichia coli Recombinant expression of AsCpf1 protein in bacteria.
[0071] The primer sequences used are shown in Table 1:
[0072] Table 1
[0073] Primer name Primer sequence (5'-3') Cpf1-2NLS-FW gggaattgtgagcggataacaattcccc SV40-RV cccactttacgtttctttttaggactgccctttttcttttt...
Embodiment 2
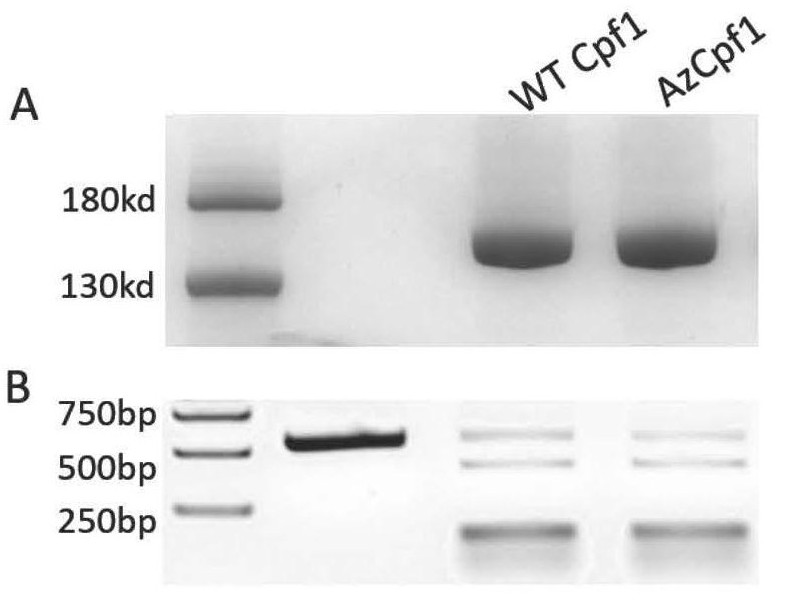
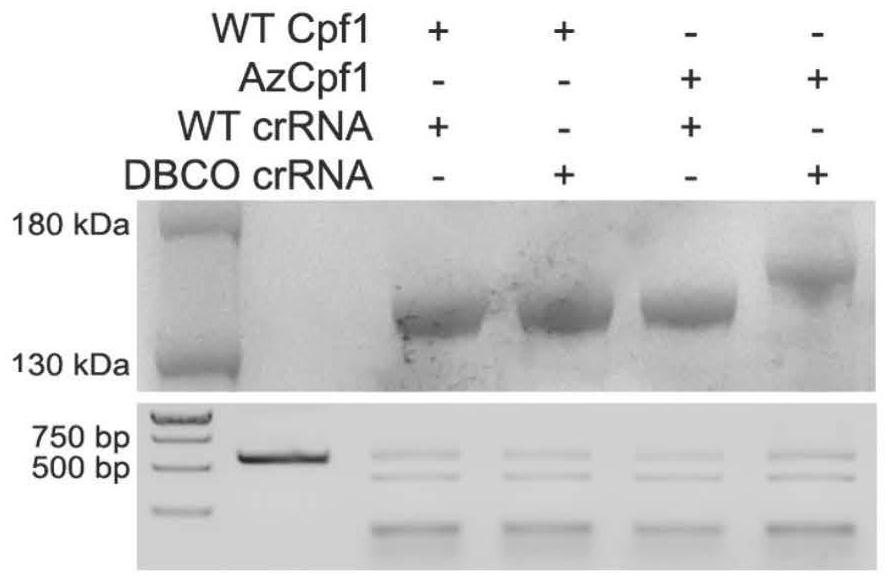
[0082] Example 2: Expression, purification and in vitro activity verification of chemically modified Cpf1 (AzCpf1)
[0083] 1: The amino acid is synthesized by the inventor
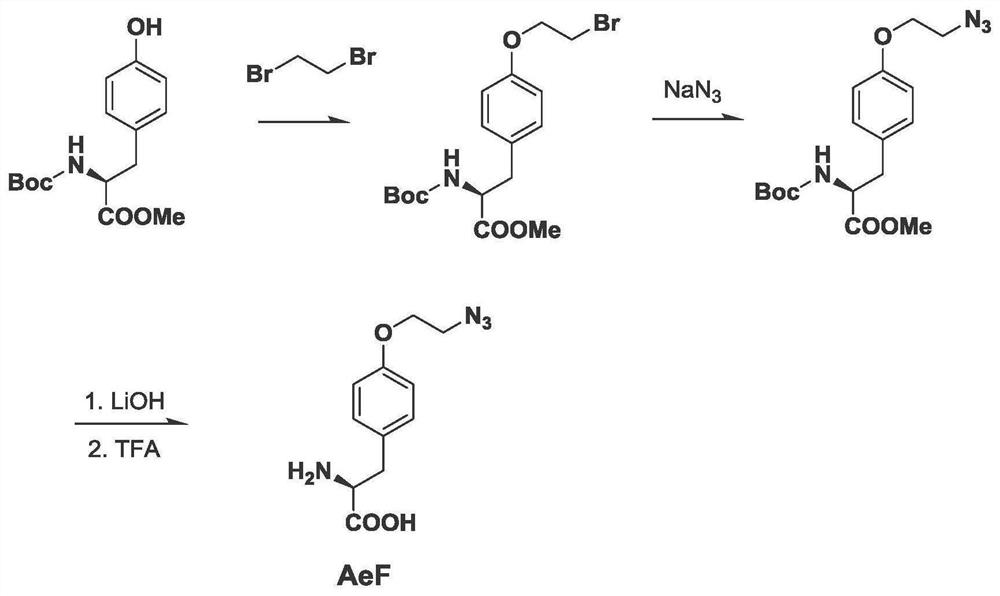
[0084] Synthetic route see figure 1 , and the synthetic steps were operated with reference to the literature.
[0085] 2: Chemically modify the expression of Cpf1 (AzCpf1) protein
[0086] The expression strain BL21-pET22b-AsCpf1-M806TAG obtained in step (4) of Example 1 was cultured overnight in a 2YT medium (containing spectinomycin and ampicillin) at 37° C. and 220 rpm in a constant temperature shaker. The next day, inoculate into fresh 2YT medium (containing spectinomycin and ampicillin) at a ratio of 1:100, culture in a constant temperature shaker at 37°C and 220 rpm, and add 1 mM AeF amino acid to continue to Cultivate, and when the OD value is 0.6-1.0, add 0.2mMIPTG (Isopropylβ-D-1-thiogalactopyranoside), and induce the expression of 16- Bacteria were collected after 18 hours. The positive con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com