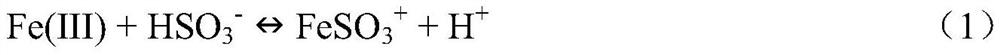Method for removing sulfite in wastewater
A sulfite and sulfite technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve problems such as high sulfate ion concentration, inability to meet national discharge standards, and inability to recycle catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A method for removing sulfite in wastewater, comprising the steps of:
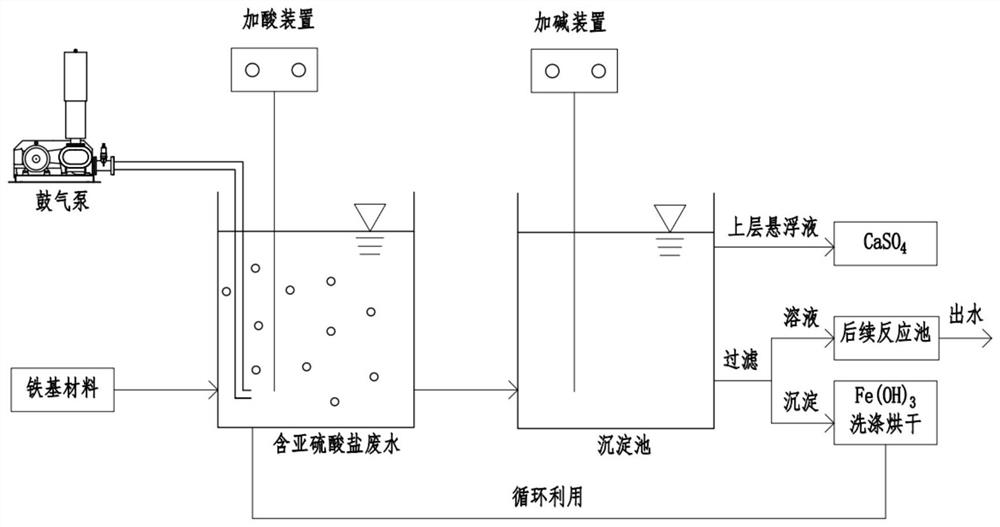
[0043] S1. If figure 1 As shown, add sulfuric acid to the sodium sulfite wastewater through the acid adding device to adjust the pH to 3, add ferric sulfate to maintain the concentration ratio of iron ions and sulfite ions in the wastewater at 1:1;
[0044] S2. Turn on the blower pump to aerate the wastewater and stir the reaction for 20 minutes, discharge the wastewater after the reaction into the sedimentation tank, add calcium hydroxide through the alkali adding device to adjust the pH of the wastewater to 8, let it settle for 2 hours, and dissolve the calcium sulfate suspension The sedimentation tank is discharged from the outlet at the upper end, and the ferric hydroxide precipitate that is insoluble in water under alkaline pH is separated from the reaction solution through the outlet at the lower end by a plate-and-frame filter or a belt filter, and the water is discharged.
[0045] The conc...
Embodiment 2
[0047] A method for removing sulfite in wastewater, comprising the steps of:
[0048] S1. If figure 1 As shown, add hydrochloric acid to the sodium sulfite wastewater through the acid adding device to adjust the pH to 2, add iron powder, and maintain the concentration ratio of iron ions and sulfite ions in the wastewater at 0.5:1;
[0049] S2. Turn on the blower pump to aerate the waste water and stir it for 50 minutes, discharge the waste water after the reaction into the sedimentation tank, add calcium hydroxide through the alkali adding device to adjust the pH of the waste water to 7, let it settle for 0.5 h, and suspend the calcium sulfate The material is discharged from the sedimentation tank from the outlet at the upper end, and the ferric hydroxide precipitate that is insoluble in water under alkaline pH is separated from the reaction solution through the outlet at the lower end by a plate-and-frame filter or a belt filter, and the water is discharged.
[0050] The conc...
Embodiment 3
[0052] A method for removing sulfite in wastewater, comprising the steps of:
[0053] S1. If figure 1 As shown, add hydrochloric acid to the potassium sulfite wastewater through the acid adding device to adjust the pH to 4, add ferric chloride to maintain the concentration ratio of iron ions and sulfite ions in the wastewater at 5:1;
[0054] S2. Turn on the blower pump to aerate the waste water and stir the reaction for 40 minutes, discharge the waste water after the reaction into the sedimentation tank, add calcium hydroxide through the alkali adding device to adjust the pH of the waste water to 9, let it settle for 6 hours, and dissolve the calcium sulfate suspension The sedimentation tank is discharged from the outlet at the upper end, and the ferric hydroxide precipitate that is insoluble in water under alkaline pH is separated from the reaction solution through the outlet at the lower end by a plate-and-frame filter or a belt filter, and the water is discharged.
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com